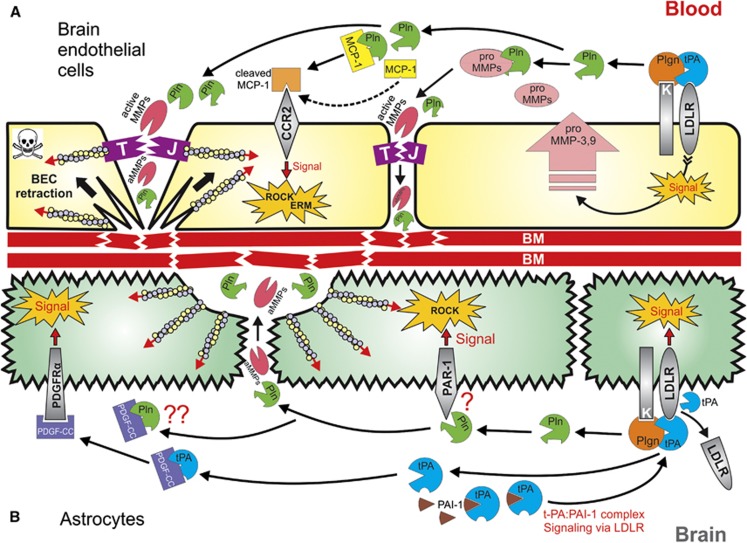
Figure 1.
Proposed integrated model for tissue-type plasminogen activator (tPA)- and plasminogen (Plgn)-mediated modulation of blood–brain barrier (BBB) permeability during thrombolysis in ischemic stroke. (A) In brain endothelial cells (BECs), tPA engages low-density lipoprotein receptor (LDLR)-related protein 1 (LRP-1) (upregulated by the ischemic insult39) and activates surface-bound plasminogen119, 126 into plasmin (Pln). LRP-1 binding by tPA signals BECs to produce and secrete matrix metalloproteinase (MMP)-339 and -9.25, 26, 62 Plasmin activates these MMPs,97, 98, 99 which in turn degrade tight junctions (TJs), increasing the paracellular permeability in a process boosted by the ischemic damage to TJs. A flux of vascular tPA, plasminogen, plasmin, and activated MMPs then flows through the broken TJs and also arrives from within the injured brain, localizing on the basolateral BEC membrane, where plasmin continues to form. Subsequently, plasmin and the MMPs degrade the basement membrane (BM),42, 69, 94, 95 causing loss of BEC adhesion126, 128 and substantial damage to the BBB. In addition, plasmin also cleaves monocyte chemoattractant protein-1 (MCP-1),150 an event that enhances the ability of MCP-1 to initiate Rho-kinase (ROCK) and ezrin–radixin–moesin (ERM) signaling via its receptor C-C chemokine receptor type 2 (CCR2).122 Activation of the ROCK and ERM pathways results in modulation of the actin cytoskeleton122 and BEC retraction,43, 119, 126, 127, 128 further enhancing TJs damage and exposure of the BM to plasmin and MMPs. Thus, the MCP-1 pathway initiates a positive feedback loop for additional loss of BEC adherence, ultimately leading to cell death and BBB impairment. (B) In astrocytes, specific tPA-mediated plasmin generation on the glial surface triggers a dual tPA-plasmin signaling events.43 These include tPA-induced cleavage and shedding of LRP-1,27 which initiates LRP-1-dependent signal, and also plasmin-mediated activation of the ROCK pathway,43 potentially via engagement of protease-activated receptor 1 (PAR-1).103, 142 LRP-1 signaling and ROCK activation in turn cause modulation of the actin cytoskeleton,43 morphology changes and end-feet retraction in astrocytes,24, 27, 38, 42, 43, 142 leading to disruption of the BBB. Tissue-type plasminogen activator also activates platelet-derived growth factor C (PDGF-CC), which signals to astrocytes via the PDGF receptor-α (PDGFRα) to impair the BBB.24 It is plausible that plasmin, known for its ability to process PDGF-CC,91, 92 may possess a similar capacity to initiate PDGF-CC-dependent BBB disruption, but this remains to be formally demonstrated.