Abstract
Stress increases the secretion of glucocorticoids (GCs), potent steroid hormones that exert their effects on numerous target tissues by acting through glucocorticoid receptors (GRs). GC signaling significantly affects ingestive behavior and taste preferences in humans and rodent models, but far less is known about the hormonal modulation of the peripheral sensory system that detects and assesses nutrient content of foods. A previous study linked restraint stress in rats to diminished expression of mRNA for one subunit of the sweet taste receptor (Tas1r3) in taste tissue and reduced gustatory nerve excitation by sweet compounds. Using RT-PCR, we detected mRNAs for GRα in circumvallate taste papillae and in oral epithelium devoid of taste buds (“non-taste” tissue). Further, circumvallate tissue was significantly enriched in GR mRNA compared to non-taste tissue based on quantitative PCR. Histologically, GR protein was expressed in all taste bud populations examined (circumvallate, foliate and fungiform papillae). Using transgenic mice expressing green fluorescent protein, almost all (97%) Tas1r3-positive taste cells (sweet-/umami-sensitive) expressed GR compared to a significantly smaller percentage (89%) of TrpM5-positive taste cells (sweet-, umami- and bitter-sensitive). When mice (n = 4) were restrain stressed, GR protein mobilized to the nucleus in Tas1r3-GFP taste cells (1.7-fold over controls). Our results suggest that GR can be activated in taste receptor cells and may play a role in specific taste qualities (e.g., sweet, umami, and bitter) to shape how the taste system responds to stress.
Keywords: Glucocorticoid receptor, Stress, Taste, Steroid hormone receptor, Taste receptor cell, Tas1r3
1. Introduction
Glucocorticoids (GCs) are potent regulators of ingestive behavior, notably food preferences and taste behavior. Feeding behavior is strongly linked to the actions of GCs via the hypothalamic–pituitary–adrenal (HPA) axis [5,21]; behavioral stress activates adrenal GC secretion while heightened HPA activity has chronic and transgenerational effects on many behavioral and physiological processes associated with feeding and metabolism [3,38]. For taste specifically, behavioral stress is known to modulate sweet and salt preferences in animal models where sweet preferences and sensitivity are significantly reduced [13,33,41]. Experimental disruption of the HPA axis (e.g., adrenalectomy) can diminish sweet preferences while increasing salt preferences, and hormone replacement (e.g., dexamethasone, deoxycorticosterone acetate) restores these preferences [11,34]. Further, humans with Addison’s disease (depleted circulating cortisol) display increased sensitivity for sweet and salty solutions that is reversed with GC treatment [14], and olfactory thresholds are also lowered in these patients suggesting a shared dysfunction in stimulus detection [15]. Thus, stress and the taste sensory system are demonstrably linked.
Glucocorticoids act through their receptors (glucocorticoid receptors α, β and γ; GRα, GRβ and GRγ, respectively) to elicit transcriptional changes in target tissues [8,39]; GRs can have rapid, nongenomic actions as well [36]. In sensory tissues, activated GRs play protective roles (e.g., prevention of acoustic damage in hair cells [24], protection against photoreceptor loss in retinal cells [40]) and may modulate sensory signaling (e.g., b-wave in the retina [1]), perhaps by activation or transrepression of gene expression. Among chemosensory epithelia, olfactory receptor neurons express GRs that, when activated by GCs, accelerate apoptosis and enhance cell regeneration [32,35]. However, the role of GRs in the taste organ and their activational capacity has not been determined, though glucocorticoid signaling plays a crucial role in feeding and metabolism.
Sweet and umami taste stimuli act on specific G protein-coupled receptors, type 1 taste receptors, encoded by Tas1r genes (Tas1r1, Tas1r2, Tas1r3). The T1r2 + T1r3 heteromer is responsive to sugars and artificial sweeteners [42], while the T1r1 + T1r3 heteromer generates an umami-sensitive taste receptor [26]. Downstream of ligand binding, the transient receptor potential channel TrpM5 facilitates cation influx and consequent depolarization of the taste cell [29]. The present study takes advantage of two transgenic mouse lines engineered to express GFP in either T1r3-expressing cells only (Tas1r3-GFP) or TrpM5-expressing cells (TrpM5-GFP), with the latter indicating the entire population of type 2 taste cells (sweet, umami, bitter) [4,7].
It is currently unknown if steroid hormones that regulate metabolism and ingestive behavior, such as glucocorticoids, target taste receptor cells in the periphery. One previous study demonstrated that chronic restraint stress (8 h/day; 14 days) diminishes Tas1r3 expression in rat taste buds and consequently reduces chorda tympani taste nerve responses to sweet and umami solutions [27]. However, it is unknown if glucocorticoid signaling underlies these changes in gene expression. Here we determined the pattern of GR expression in the taste buds of mice and whether GR can be activated by behavioral stress.
2. Materials and methods
2.1. Animals
C57BL/6 (wild type) and Tas1r3-GFP and TrpM5-GFP transgenic mice (>4 months old; both sexes) were used. Mice were euthanized prior to removal of tongues for isolation of taste tissues (circumvallate papillae [CV], foliate papille [FOL], fungiform papillae [FUNG], non-taste epithelium [NT]) and control tissues (adrenal, adipose). CV tissue from Skn-1a knockout (KO) mice (−/− and +/−) was provided by Matsumoto [23].
The care and euthanization of mice in this study was in accordance with NIH Guidelines for the Care and Use of Laboratory Animals. All of the experimental procedures involving mice in this study were approved by the Institutional Animal Care and Use Committee at Monell (IACUC ACC#1155).
2.2. RT-PCR and quantitative PCR
Excised tongues from C57BL/6 male mice (n = 13) and female mice (n = 5) were injected with an enzyme mix (collagenase I [1 mg/ml], dispase II [2 mg/ml]; Sigma) diluted in calcium-free Hank’s buffered saline solution (Life Technologies). Taste epithelia and control non-taste epithelial peels (ventral surface devoid of taste buds) were separated before infiltrating with RNA later overnight at (4 °C) and freezing (−80 °C). RNA was isolated using PureLink Mini Columns (Life Technologies) according to the manufacturer’s instructions with on-column DNaseI treatment (Invitrogen). Samples were purified (RNA Clean and Concentrator kit; Zymo Research) prior to concentration determination on a Nanodrop (Thermo Scientific). Individual cDNAs were synthesized from 100 ng of total RNA using the VILO kit (Invitrogen). cDNA quality was assessed by RT-PCR using intron-spanning control primers (GAPDH) with the Superscript III kit (Invitrogen). Amplification products were visualized using gel electrophoresis (2% agarose with ethidium bromide [Fisher Scientific]). The first round of no-reverse transcriptase controls was negative for β-actin bands (Supplementary Fig. S1), thus these controls were run only once to conserve taste RNA for the remaining samples.
Supplementary figure related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2014.04.047.
RT-PCR reactions were then carried out for CV and FOL papillae and NT epithelium in addition to control tissues (adrenal, adipose) as per the manufacturer’s instructions (Superscript III, Invitrogen). The primer sets were: β-actin, (F: GGCTGTATTCCCCTCCATCG, R: CCAGTTGGTAACAATGCCATGT), GAPDH (F: CCTTCATTGACCTCAACTAC, R: GGAAGGCCATGCCAGTGAGC), GR (F: AACCTGGATGACCAAATGACC, R: GCAGGTTTCCACTTGCTTGT).
Quantitative PCR primers for GR (Nr3c1), α-gustducin (Gnat3) and T1r3 (Tas1r3) were either modified from published primer sets or obtained from PrimerBank: Nr3c1 (same as above), Gnat3 (F: TAGGAGCCGAGAGGACCAAG, R: GCTGGTATTCAGATGCCCTTTC), Tas1r3 (F: CAGGCAGTTGTGACTCTGTTG, R: TGCGATGCAGATACCTCGTG). Reactions (0.5 μl cDNA each) were run in triplicate (Fast SYBR Green protocol; AB Biosystems) with melting curves for every reaction (Step-One Plus machine). Values (Ct) were normalized to β-actin expression levels for each sample (ΔCt method [30]).
2.3. Immunohistochemistry
Tongues were fixed in 4% paraformaldehyde for 2 h at 4 °C then transferred to 20% sucrose (overnight, 4 °C). Taste tissues (CV, FOL, fungiform [FUNG]) were embedded in OCT medium (EMS) and frozen. Cryostat sections (10 μm) were placed on glass slides sequentially such that sections were at least 70 μm apart in depth per slide to avoid recounting taste cells. Slides were blocked (Superblock; Thermo) for 3 h (room temperature; RT) before adding primary antibodies (rabbit anti-GR, sc-1004[M-20]; goat anti-T1R3, sc-22458[N-20], Santa Cruz) overnight at 4 °C and secondary antibodies (donkey-anti-rabbit Alexa Fluor 594; donkey-anti-goat Alexa Fluor 488; 1:800; Invitrogen) for 1.5 h in the dark (RT). Nuclei were stained (DAPI, 1:10,000) before mounting (VectaShield). Tissues were visualized with confocal laser microscopy (Leica SP3 system).
The GR antibody was validated by omitting primary antibody and by incubating primary with blocking peptide (Supplementary Fig. S2A–C). We further validated the nature of GR immunoreactivity by testing tongue sections from Skn-1a KO mice that lack all type 2 taste cells [23].
Supplementary figure related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2014.04.047.
Taste buds from Tas1r3-GFP mice (n = 5) and TrpM5-GFP mice (n = 6) were assessed for coexpression of GFP with immunoreactivity to GR. The number of GFP-positive cells containing nuclei (DAPI stain) was counted in Photoshop (Adobe) before counting antibody-positive cells. Percent coexpression was calculated as the number of GFP-positive cells expressing GR divided by the number of GFP-positive cells per taste bud. At least four sections per animal were scored for coexpression in CV, FOL and FUNG tissues from mice.
2.4. Restraint stress
To determine if stress could alter expression of GR within taste receptor cells we stressed male Tas1r3-GFP mice (n = 4; 2 h) by using a standard restraint stress protocol [28]. Briefly, stressed animals were immobilized in conical tubes without access to food or water while control mice (n = 4) were food and water deprived simultaneously for 2 h before processing taste tissues for immunohistochemistry.
An observer (DF) scored taste buds for nuclear expression of GR and was kept blind to experimental conditions while scoring. Taste tissue sections from control and stressed mice were placed on the same slides to avoid differences in staining intensity. The percentage of Tas1r3-GFP cells exhibiting nuclear GR staining was determined per bud per section and averaged per animal.
2.5. Statistics
Relative gene expression values were compared for genes between CV and NT tissues using paired t-tests, and coexpression of GR with Tas1r3-GFP vs. TrpM5-GFP was determined using t-tests (SigmaPlot). Nuclear expression of GR between stress and control animals was also determined with t-tests. α was set at 0.05.
3. Results
3.1. Expression of GR mRNA in taste tissue
By RT-PCR both taste and non-taste tissues expressed mRNA for GR with no apparent difference in expression in males vs. females detected (Fig. 1A and B). The Nr3c1 gene has multiple splice variants that give rise to all known GRs with the dominant form being GRα, so full length GRα mRNA was amplified from the same cDNA samples in a second round of RT-PCR reactions using previously published primers specific to GRα [16]. GRα mRNA was expressed in both taste and non-taste tissues with no apparent sexual dimorphism (Supplementary Fig. S3). When tissue-specific expression of GR was assessed in qPCR, CV was significantly enriched in GR mRNA compared to NT tissue (1.39 fold ± 0.06 S.E.M.; paired t12 = 5.11, P < 0.001) (Fig. 1C). NT tissue showed no expression of taste-specific mRNAs.
Fig. 1.
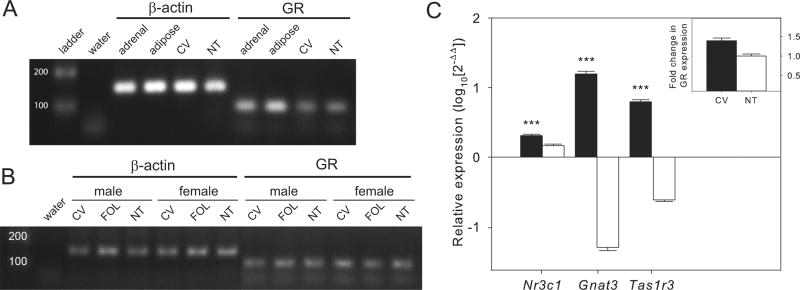
Expression of glucocorticoid receptor (GR) mRNAs in taste and other tissues from mice using RT-PCR. (A) Both circumvallate (CV) taste papillae and non-taste (NT) tissue express GR mRNAs. The water lane is a negative control, adrenal and adipose tissues from mice were used as positive tissue controls for GR expression and β-actin is a positive control gene. (B) Expression of GR was seen in all taste (CV, foliate papillae [FOL]) and NT tissues in both sexes. (C) Gene expression of GR (Nr3c1) is enriched in CV compared to NT based on quantitative PCR (n = 13 mice). Gnat3 and Tas1r3 are positive control genes for taste tissues. Gene expression was relative to control gene expression (β-actin) within each sample. Inset shows fold change in GR expression for CV compared to NT. Bars represent average ± SEM, asterisks indicate statistically significant differences (P < 0.001).
Supplementary figure related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2014.04.047.
3.2. Expression of GR protein in taste receptor cells
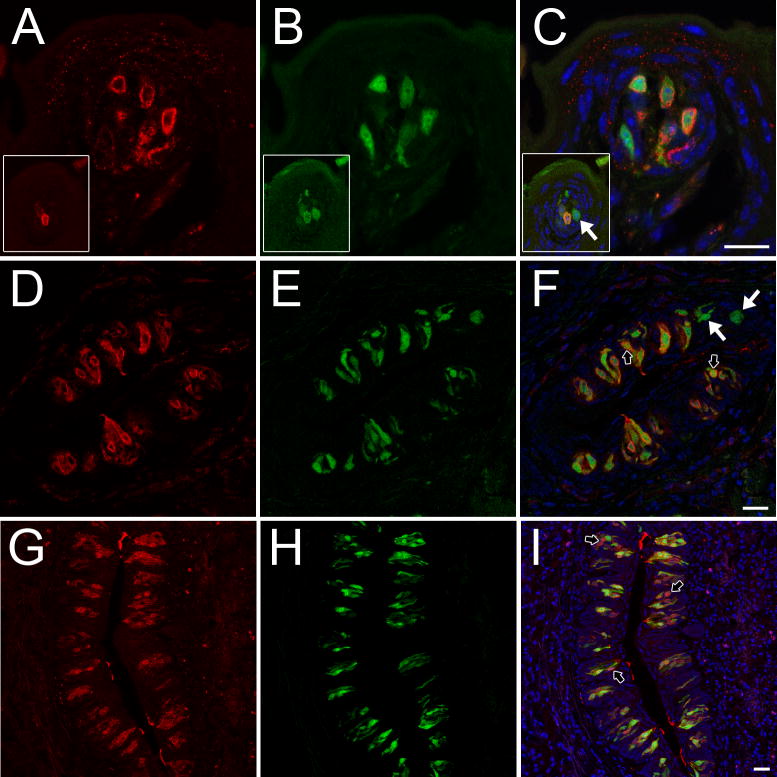
Taste receptor cells in both anterior and posterior tongue expressed GR protein (Fig. 2). GR was expressed predominantly in type 2 taste receptor cells (Supplementary Table S1). In posterior tongue, the vast majority of Tas1r3-GFP taste cells expressed GR (mean percent coexpression was 96.7% ± 1.5 in CV and 98.2% ± 0.6 in FOL). Double immunohistochemistry in wild type mice verified that all T1r3 immunopositive taste cells expressed GR (Supplementary Fig. S4). However, many taste buds had cells expressing GR but not T1r3 indicating that the set of GR-positive taste cells is larger than and fully contains the set of T1r3-positive taste cells. To determine if GR-positive/T1r3-negative taste cells were type 2 cells we used a TrpM5-GFP reporter line where all type 2 taste cells express GFP [4]. All GR-positive taste cells were TrpM-GFP-positive. Most TrpM5-GFP taste cells were immunopositive for GR (87.2% ± 1.6; t7 = 4.192, P = 0.004 in CV and 93.0% ± 1.2; t9 = 3.501, P = 0.007 in FOL) (Fig. 2, D–F), although the percentages were significantly smaller than those observed for Tas1r3-GFP cells expressing GR. There was no GR immunoreactivity in taste receptor cells of Skn-1a KO mice that lack all type 2 taste cells (Supplementary Fig. S2D–F). Thus, all GR immunoreactivity in taste buds is within type 2 taste cells, although most but not all type 2 cells show GR immunoreactivity.
Fig. 2.
Expression of GR protein (red) in T1r3- and TrpM5-expressing taste receptor cells containing a green fluorescent protein (GFP) reporter gene (green) in mouse taste tissues. Blue is a nuclear stain (DAPI). (A–C) GR is expressed in all Tas1r3-GFP cells in fungiform papillae but not in all TrpM5-GFP cells (insets; white arrow). (D–F) GR is not expressed in all TrpM5-GFP cells in FOL (white arrows). Black arrows indicate nuclear expression of GR. (G–I) GR is expressed in the majority of Tas1r3-GFP taste cells in CV. Black arrows indicate nuclear expression of GR. Scale bars = 20 μm. Abbreviations are as in Fig. 1.
Supplementary table related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2014.04.047.
Supplementary figure related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2014.04.047.
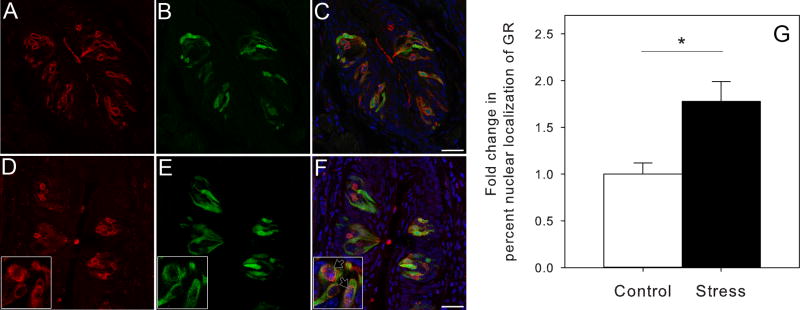
3.3. Stress induces nuclear translocation of GR in taste receptor cells
In control animals, 36.5% ± 4.3 of Tas1r3-GFP taste cells in CV showed nuclear expression of GR. Restraint stress increased nuclear expression 1.77 fold to an average nuclear expression of 64.9% ± 7.7 (Fig. 3). This increase was statistically significant (t6 = −3.191, P = 0.019). To provide physiological relevance for GR activation in sweet/umami taste cells, we treated mice with dexamethasone and saw reduced consumption of 0.5% and 1.0% sucrose solutions compared to controls in two-bottle preference tests (Supplementary Fig. S5).
Fig. 3.
Taste tissue from control and restraint stress animals showed different patterns of GR protein expression in Tas1r3-GFP taste cells. (A–C) Expression of GR (red) is mostly perinuclear in Tas1r3-GFP taste cells (green) in CV tissue from unstressed control mice based on immunohistochemistry. Blue is a nuclear stain (DAPI). (D–F) When Tas1r3-GFP mice were restrain stressed (2 h) GR expression was localized mostly to the nucleus in Tas1r3-GFP taste cells (black arrows in inset). (G) Restraint stress caused a 1.7-fold increase in percent nuclear expression of GR protein in Tas1r3-GFP CV of mice (n = 4) compared to unstressed controls (n = 4). Bars represent means ± SEM. Asterisk represents a statistically significant difference (P < 0.05). Abbreviations are as in Fig. 1.
Supplementary figure related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2014.04.047.
4. Discussion
Our data provide the first description of a steroid hormone receptor and its potential activation in the peripheral taste organ. GR is expressed in all taste bud populations: CV, FOL and FUNG. GR is specifically expressed in type 2 taste receptor cells, especially T1r3-expressing taste cells known to respond to sweet and umami stimuli. Lastly, translocation of GR to the nucleus in mice is inducible by behavioral stress and provides a potential mechanism for activation of particular genes in taste receptor cells by GR’s actions.
In terms of protein expression patterns, GR was localized to the perinuclear space of taste cells, consistent with what has been seen in another sensory tissue (vestibular cells of the inner ear, [37]). GR translocated to the nucleus following restraint stress in our experiment similar to GR in guinea pig cochlear cells following GC treatment [19]. GR was sometimes apically expressed which may represent a pathway for free steroid signaling from salivary sources, though most salivary steroids are bound by proteins [12]. GC signaling in taste buds, as in other sensory tissues, may rescue sensory cells from damage or accelerate cell turnover [24,40]. GCs also may enhance peripheral sensitivity to the same stimuli via repression or enhancement of gene expression in taste receptor cells.
Taste receptor cells are important targets of hormones regulating metabolism and consummatory behavior (reviewed in [43]). Leptin receptors are expressed by taste receptor cells [18], and endocannabinoids oppose the actions of leptin on sweet taste cells by acting on CB(1) receptors [44]. Steroid hormones have well known metabolic effects on target tissues, and GCs especially have specific effects on feeding behavior that are modulated by the consumption of sweet foods. Nutritive sweet solutions dampen or sometimes block stress-induced effects on feeding behavior in rodents by acting directly on the HPA axis [6], and self-reported stress levels are decreased in men given high calorie meals following stress [22]. Thus, the consumption of sweet foods during stress strengthens negative feedback on the HPA axis, and how GCs modulate the peripheral taste environment is a fruitful research question.
In humans, stress and GCs have a variety of effects on taste perception and sensitivity. Emotional and physical stress can reduce the duration and intensity of taste experiences [2,25,31]. Stress effects on eating can be sex-specific, such as women increasing their consumption of sweet foods following stress [9,45]. This increase may be due to reduced sensitivity to sweet stimuli [2], though sweet taste thresholds in humans can decrease immediately following acute stress exposure [17]. Other tastes are also affected by GCs, such as salt taste thresholds in men that increase following administration of hydrocortisone [10]. For fat preference, inhibition of GC signaling (via RU 486) has no effect, suggesting that the effect of GCs on taste perception may be specific to certain taste stimuli [20]. Taken together, the role that stress hormones may play in regulating human taste remains elusive, but the existing data combine with our results to demonstrate that stress hormones may act directly on the peripheral taste organ.
Supplementary Material
HIGHLIGHTS.
GR mRNA and protein were expressed in mouse taste buds.
CV taste tissue was enriched in GR mRNA compared to non-taste epithelium.
GR was coexpressed with the T1r3 sweet/umami taste receptor subunit in taste buds.
Restraint stress induced nuclear localization of GR in taste receptor cells.
Acknowledgments
The authors would like to thank I. Matsumoto and M. Ohmoto for donating tissue from Skn-1aKO mice. This work was partially supported by DC000014 (MRP), DC003055 (RFM) and an NIH-NIDCD Diversity Supplement (BC). Aspects of the work were performed at P30 Core facilities at Monell funded by NIH-NIDCD 1P30DC011735-02.
References
- 1.Abraham I, Palhalmi J, Szilagyi N, Juhasz G. Glucocorticoids alter recovery processes in the rat retina. NeuroReport. 1998;9:1465–1468. doi: 10.1097/00001756-199805110-00040. [DOI] [PubMed] [Google Scholar]
- 2.Al’Absi M, Nakajima M, Hooker S, Wittmers L, Cragin T. Exposure to acute stress is associated with attenuated sweet taste. Psychophysiology. 2012;49:96–103. doi: 10.1111/j.1469-8986.2011.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 4.Clapp T, Medler K, Damak S, Margolskee R, Kinnamon S. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallman MF. Stress update: adaptation of the hypothalamic–pituitary–adrenal axis to chronic stress. Trends Endocrinol Metabol. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- 6.Dallman MF, Akana SF, Laugero KD, Gomez F, Manalo S, Bell ME, Bhatnagar S. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79:3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 7.Damak S, Mosinger B, Margolskee R. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008;9:96. doi: 10.1186/1471-2202-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10:2807–2816. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- 9.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 10.Fehm-Wolfsdorf G, Scheible E, Zenz H, Born J, Fehm HL. Taste thresholds in man are differentially influenced by hydrocortisone and dexamethasone. Psychoneuroendocrinology. 1989;14:433–440. doi: 10.1016/0306-4530(89)90042-5. [DOI] [PubMed] [Google Scholar]
- 11.Fregly MJ, Rowland NE. Role of renin-angiotensin-aldosterone system in NaCl appetite of rats. Am J Physiol. 1985;248:R1–R11. doi: 10.1152/ajpregu.1985.248.1.R1. [DOI] [PubMed] [Google Scholar]
- 12.Hammond GL, Langley MS. Identification and measurement of sex hormone binding globulin (SHBG) and corticosteroid binding globulin (CBG) in human saliva. Acta Endocrinol (Copenh) 1986;112:603–608. doi: 10.1530/acta.0.1120603. [DOI] [PubMed] [Google Scholar]
- 13.Harkin A, Houlihan DD, Kelly JP. Reduction in preference for saccharin by repeated unpredictable stress in mice and its prevention by imipramine. J Psychopharmacol (Oxf) 2002;16:115–123. doi: 10.1177/026988110201600201. [DOI] [PubMed] [Google Scholar]
- 14.Henkin RI, Gill JR, Barrter FC. Studies on taste thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids and of serum sodium concentration. J Clin Invest. 1963;42:727–735. doi: 10.1172/JCI104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henkin RI, Barrter FC. Studies on olfactory thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids and of serum sodium concentration. J Clin Invest. 1966;45:1631–1639. doi: 10.1172/JCI105470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinds TD, Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, Sanchez ER. Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol Endocrinol. 2010;24:1715–1727. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ileri-Gurel E, Pehlivanoglu B, Dogan M. Effect of acute stress on taste perception: in relation with baseline anxiety level and body weight. Chem Senses. 2013;38:27–34. doi: 10.1093/chemse/bjs075. [DOI] [PubMed] [Google Scholar]
- 18.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kil SH, Kalinec F. Expression and dexamethasone-induced nuclear translocation of glucocorticoid and mineralocorticoid receptors in guinea pig cochlear cells. Hear Res. 2013;299:63–78. doi: 10.1016/j.heares.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramlik SK, Altemus M, Castonguay TW. The effects of the acute administration of RU 486 on dietary fat preference in fasted lean and obese men. Physiol Behav. 1993;54:717–724. doi: 10.1016/0031-9384(93)90082-q. [DOI] [PubMed] [Google Scholar]
- 21.Laugero KD. A new perspective on glucocorticoid feedback: relation to stress, carbohydrate feeding and feeling better. J Neuroendocrinol. 2001;13:827–835. doi: 10.1046/j.1365-2826.2001.00706.x. [DOI] [PubMed] [Google Scholar]
- 22.Macht M. Effects of high- and low-energy meals on hunger, physiological processes and reactions to emotional stress. Appetite. 1996;26:71–88. doi: 10.1006/appe.1996.0006. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14:685–687. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meltser I, Canlon B. Protecting the auditory system with glucocorticoids. Hear Res. 2011;281:47–55. doi: 10.1016/j.heares.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa M, Mizuma K, Inui T. Changes in taste perception following mental or physical stress. Chem Senses. 1996;21:195–200. doi: 10.1093/chemse/21.2.195. [DOI] [PubMed] [Google Scholar]
- 26.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJP, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto A, Miyoshi M, Imoto T, Ryoke K, Watanabe T. Chronic restraint stress in rats suppresses sweet and umami taste responses and lingual expression of T1R3 mRNA. Neurosci Lett. 2010;486:211–214. doi: 10.1016/j.neulet.2010.09.055. [DOI] [PubMed] [Google Scholar]
- 28.Pare WP, Glavin GB. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev. 1986;10:339–370. doi: 10.1016/0149-7634(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 29.Pérez CA, Margolskee RF, Kinnamon SC, Ogura T. Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium. 2003;33:541–549. doi: 10.1016/s0143-4160(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rai B, Kaur J. Mental and physical workload, salivary stress biomarkers and taste perception: Mars desert research station expedition. North Am J Med Sci. 2012;4:577–581. doi: 10.4103/1947-2714.103318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson AM, Kern RC, Foster JD, Fong KJ, Pitovski DZ. Expression of glucocorticoid receptor mRNA and protein in the olfactory mucosa: physiologic and pathophysiologic implications. Laryngoscope. 1998;108:1238–1242. doi: 10.1097/00005537-199808000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Sampson D, Muscat R, Phillips G, Willner P. Decreased reactivity to sweetness following chronic exposure to mild unpredictable stress or acute administration of pimozide. Neurosci Biobehav Rev. 1992;16:519–524. doi: 10.1016/s0149-7634(05)80193-9. [DOI] [PubMed] [Google Scholar]
- 34.Silva MT. Saccharin aversion in the rat following adrenalectomy. Physiol Behav. 1977;19:239–244. doi: 10.1016/0031-9384(77)90332-8. [DOI] [PubMed] [Google Scholar]
- 35.Takanosawa M, Nishino H, Ohta Y, Ichimura K. Glucocorticoids enhance regeneration of murine olfactory epithelium. Acta Otolaryngol (Stockh) 2009;129:1002–1009. doi: 10.1080/00016480802530663. [DOI] [PubMed] [Google Scholar]
- 36.Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity. 2006;14:259S–265S. doi: 10.1038/oby.2006.320. [DOI] [PubMed] [Google Scholar]
- 37.Terakado M, Kumagami H, Takahashi H. Distribution of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase isoforms in the rat inner ear. Hear Res. 2011;280:148–156. doi: 10.1016/j.heares.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300:137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 39.Webster JC, Cidlowski JA. Mechanisms of glucocorticoid-receptor-mediated repression of gene expression. Trends Endocrinol Metabol. 1999;10:396–402. doi: 10.1016/s1043-2760(99)00186-1. [DOI] [PubMed] [Google Scholar]
- 40.Wenzel A, Grimm C, Seeliger MW, Jaissle G, Hafezi F, Kretschmer R, Zrenner E, Reme CE. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001;42:1653–1659. [PubMed] [Google Scholar]
- 41.Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida R, Niki M, Jyotaki M, Sanematsu K, Shigemura N, Ninomiya Y. Modulation of sweet responses of taste receptor cells. Semin Cell Dev Biol. 2013;24:226–231. doi: 10.1016/j.semcdb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida R, Ohkuri T, Jyotaki M, Yasuo T, Horio N, Yasumatsu K, Sanematsu K, Shigemura N, Yamamoto T, Margolskee RF, Ninomiya Y. Endocannabinoids selectively enhance sweet taste. Proc Natl Acad Sci U S A. 2010;107:935–939. doi: 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiol Behav. 2006;87:789–793. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.