Abstract
Contact dermatitis (CD) is a pattern of inflammatory responses in the skin that occurs through contact with external factors. The clinical picture is a polymorphic pattern of skin inflammation characterized by a wide range of clinical features, including itching, redness, scaling, and erythema. Coriandrum sativum L. (CS), commonly known as coriander, is a member of the Apiaceae family and is cultivated throughout the world for its nutritional and culinary values. Linoleic acid and linolenic acid in CS have various pharmacological activities. However, no study of the inhibitory effects of CS on CD has been reported. In this study, we demonstrated the protective effect of CS against 2,4-dinitrochlorobenzene-induced CD-like skin lesions. CS, at doses of 0.5–1%, applied to the dorsal skin inhibited the development of CD-like skin lesions. Moreover, the Th2-mediated inflammatory cytokines, immunoglobulin E, tumor necrosis factor-α, interferon-γ, interleukin (IL)-1, IL-4, and IL-13, were significantly reduced. In addition, CS increased the levels of total glutathione and heme oxygenase-1 protein. Thus, CS can inhibit the development of CD-like skin lesions in mice by regulating immune mediators and may be an effective alternative therapy for contact diseases.
Key Words: : 2,4-dinitrochlorobenzene; contact dermatitis-like skin; Coriandrum sativumL.; scratching behavior; Th2-mediated cytokines
Introduction
Allergic diseases, including contact dermatitis (CD), are chronic, relapsing, and noninfectious conditions, caused by a complex interrelationship among genetic, environmental, pharmacological, psychological, immunological, and skin barrier dysfunction factors.1 Skin affected by CD has a characteristic morphology and age-related distribution pattern and is associated with pruritus.2 CD lesions include erythematous, edematous papules, and plaques with scaling, whereas chronic lesions appear as lichenified plaques with increased skin lesions on the face and flexural areas.2,3 Specific immune and inflammatory mechanisms culminate in a complex series of cellular interactions leading to the signs and symptoms of CD.4 Acute CD skin lesions indicate the important role of Th2-type inflammation, including the secretion of interleukin (IL)-4, IL-5, and IL-13, whereas in chronic CD lesions, Th1-type immune responses, such as interferon (IFN)-γ expression by Th1 cells and delayed-type hypersensitivity reactions, are responsible.5,6 Most CD patients present with peripheral blood eosinophilia and high serum immunoglobulin (Ig) E levels due to the activation of B cells by IgE, mediated by IL-4 upregulation.7,8
Coriandrum sativum L. (CS; coriander) is an annual herb that is a member of the carrot family and is cultivated extensively in Europe and Asia.9 CS is commonly eaten as a food seasoning and the seeds are used as a spice.9 Previous studies reported that leaves of CS decreased lipopolysaccharide-induced nitric oxide (NO) production and had a NO scavenging effect in RAW 264.7 macrophages.10 Moreover, it has anti-inflammatory potential in ultraviolet erythema and antioxidant effects in rats fed a high-fat diet.11 Furthermore, we previously demonstrated that linoleic acid and linolenic acid in CS protect human skin keratinocytes against H2O2-induced oxidative damage, possibly by regulating antioxidative defense enzymes such as heme oxygenase-1 (HO-1) and total glutathione (GSH) through nuclear factor erythroid-derived 2-related factor 2.12 However, no study of the inhibitory effects of CS on CD has been reported. Therefore, this study examined the protective effects of CS against 2,4-dinitrochlorobenzene (DNCB)-induced CD-like skin lesions using clinical skin severity scores, scratching behavior, and histopathological staining in mice. We also investigated possible CS mechanisms by assessing the levels of IgE, tumor necrosis factor-α (TNF-α), IFN-γ, IL-1, IL-4, IL-13, HO-1, and GSH.
Materials and Methods
Chemical
DNCB, acetone, and olive oil were obtained from Sigma-Aldrich (Milwaukee, WI, USA). The enzyme-linked immunosorbent assay (ELISA) kits for IgE, IFN-γ, and IL-1, IL-4, and IL-13 were obtained from the R&D Systems (Minneapolis, MN, USA) and BD Biosciences (San Diego, CA, USA). All chemicals and solvents were of the highest grade commercially available.
Preparation of the CS extract and standardization
The CS extract (CSE) was prepared and standardized using a gas chromatography according to previously published methods.12 Briefly, 100 g of CS leaves was ground with 1 L of 70% ethanol in a blender for 5 min, and this was stirred for 24 h at room temperature. Then, the extract was lyophilized (yield: 4.00%). CSE was standardized based on fatty acid content determined by using gas chromatography. Palmitic acid, linoleic acid, and linolenic acid were found in CSE at mean levels of 8.57, 14.28, and 17.91 mg/g, respectively.12
Animals and treatments
Animal maintenance and treatments were carried out in accordance with the Principles of Laboratory Animal Care (NIH publication No. 85-23, revised 1985) and the Use Guidelines of Kyung Hee University, Seoul, Korea. Male ICR mice (8 weeks, 23–24 g) were purchased from the Daehan Biolink Co., Ltd. (Eumseong, Korea). The animals were housed at an ambient temperature of 23°C±1°C and relative humidity of 60%±10% under a 12-h light–12-h dark cycle and were allowed free access to water and food. The mice were assigned into five groups: (1) control group (n=5), (2) DNCB group (n=5), (3) DNCB+0.5% CSE group (n=5), (4) DNCB+1% CSE group (n=5), and (5) DNCB+dexamethasone group (n=5). CD-like skin lesions were induced by applying DNCB to the dorsal skin. After complete removal of the dorsal hairs within an area of approximately 8 cm2, 200 μL of 1% DNCB solution (dissolved in a 3:1 mixture of acetone and olive oil) was applied for 7 consecutive days for sensitization. Seven days after sensitization, the dorsal skin was challenged with 200 μL of 0.5% DNCB solution three times per week for 4 weeks. After inducing CD, the CSE solution (dissolved in a 3:1 mixture of acetone and olive oil) was applied to the dorsal skin of the mice seven times per week for 4 weeks. In the control and DNCB-alone treated mice, no CSE solution was applied to the dorsal skin. The animals were sacrificed 9 weeks after sensitization with DNCB.
Evaluation of clinical skin severity scores
The five signs of skin lesions were (1) pruritus/itching, (2) erythema/hemorrhage, (3) edema, (4) excoriation/erosion, and (5) scaling/dryness. The above-mentioned symptoms were graded as follows: 0 (no symptoms), 1 (mild), 2 (moderate), and 3 (severe).
Evaluation of scratching behavior
Mice were placed individually in clear plastic cages and their behavior was monitored for 30 min, 5 weeks after sensitization. Scratching of the rostral back and biting of the caudal back were observed; scratching movements by the hind paw were defined as a scratching bout that ended when the mice either licked its hind paw or placed its hind paw back on the floor, and a series of one or more biting movements were counted as one episode that ended when the rat returned to the straightforward position.
Evaluation of histological examination using hematoxylin and eosin
The dorsal skin of each mouse was removed and fixed in 10% formalin. Serial 20-μm-thick coronal sections were cut on a freezing microtome (Leica Instruments GmbH, Nussloch, Germany) designed for histological analysis using hematoxylin and eosin (H&E).
Evaluation of total serum IgE, TNF-α, IFN-γ, IL-1, IL-4, and IL-13 levels
Blood was collected from the mice on the day of sacrifice and centrifuged at 1700 g for 10 min to obtain serum samples which were stored at −70°C until use. Total serum IgE, TNF-α, IFN-γ, IL-1, IL-4, and IL-13 were measured using an ELISA kit according to the manufacturer's instructions.
Western blot analysis
The skin tissues were frozen in liquid nitrogen and powdered with a mortar and pestle and lysed using a cell lysis buffer. The lysates were separated on 15% SDS-PAGE and were then transferred to a membrane. The membranes were incubated with 5% skim milk in TBST for 1 h. Then, they were incubated with rabbit anti-HO-1 (1:500 dilutions) primary antibody overnight at 4°C, followed by incubation with HRP-conjugated anti-rabbit IgG for 1 h, respectively. Immunoreactive bands were detected using an ECL detection kit, and an LAS-4000 mini system (Fujifilm Corporation, Tokyo, Kumamoto, Japan) was used for visualization.
Measuring the total GSH level
Total GSH was determined using a total GSH quantification kit (Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer's directions.
Statistical analysis
All statistical parameters were calculated using the Graphpad Prism 4.0 software. Values were expressed as the mean±standard error of the mean. The results were analyzed by one-way analysis of variance. Differences with a P-value less than .05 were considered statistically significant.
Results
Effects of CSE on clinical skin severity scores
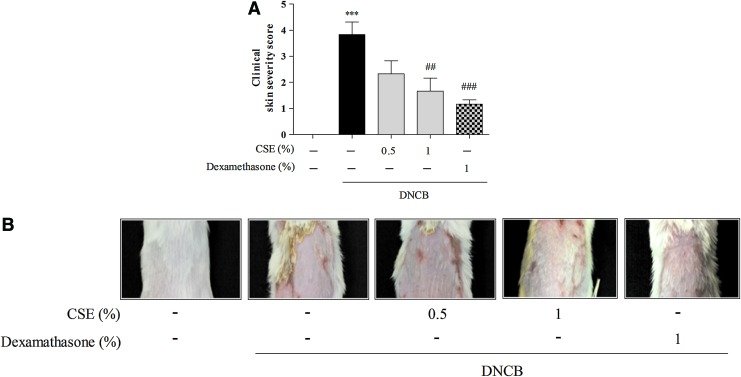
To investigate the protective effects of CSE against DNCB-induced CD-like skin damage, we measured clinical skin severity scores. Treatment with DNCB significantly increased clinical skin severity scores compared with control cells (by 3.83%±0.48%), whereas treatment with 0.5 and 1% CSE or 1% dexamethasone reduced DNCB-induced clinical skin severity scores (by 2.33%±0.49%–1.67±0.48% and 1.17%±0.17%, respectively) (Fig. 1).
FIG. 1.
The effect of Coriandrum sativum L. extract (CSE) on 2,4-dinitrochlorobenzene (DNCB)-induced clinical skin severity scores in mice. Nine weeks after the initial sensitization, the clinical skin severity scores of the DNCB-treated mice were assessed. Clinical skin severity scores were evaluated as the sum of the scores for five clinical symptoms (A). Representative images are shown (B). Values are means±standard error of the mean. ***P<.001 compared with the control group; ##P<.01 and ###P<.001 compared with the DNCB-alone group. Color images available online at www.liebertpub.com/jmf
Effects of CSE on scratching behavior
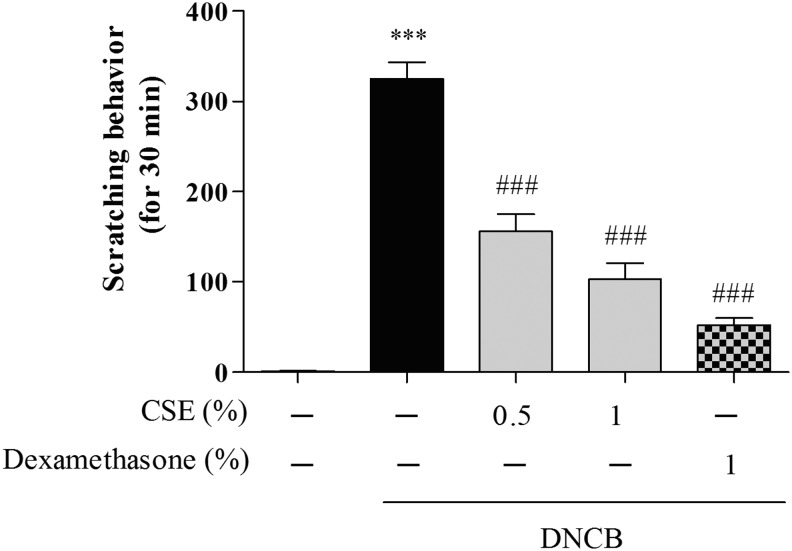
To investigate the protective effects of CSE against DNCB-induced CD-like skin damage, we measured scratching behavior. Treatment with DNCB significantly increased scratching scores compared with control cells (by 325.01%±14.89%), whereas treatment with 0.5 and 1% CSE or 1% dexamethasone decreased DNCB-induced scratching scores (by 156.10%±15.76%–103.02%±14.17% and 52.33%±6.52%, respectively) (Fig. 2).
FIG. 2.
The effect of CSE on DNCB-induced scratching behavior. Nine weeks after the initial sensitization, the clinical scores of the DNCB-treated mice were assessed. The number of scratching behaviors within 30 min was measured 1 h after each sample treatment. Values are means±standard error of the mean. ***P<.001 compared with the control group; ###P<.001 compared with the DNCB-alone group.
Effects of CSE on histological parameters
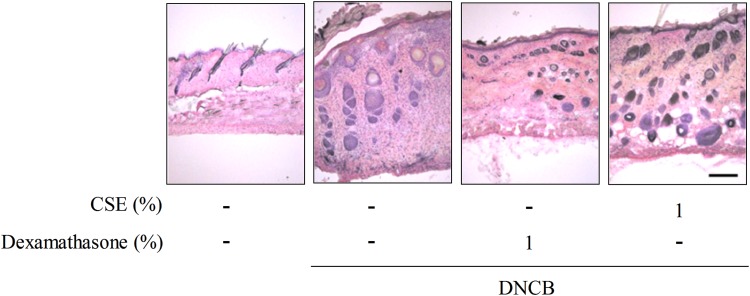
To investigate the protective effects of CSE against DNCB-induced CD-like skin damage, we performed H&E staining. Treatment with DNCB significantly increased skin thickness, whereas treatment with CSE or dexamethasone reduced DNCB-induced skin thickness (Fig. 3).
FIG. 3.
The effect of CSE on DNCB-induced histopathological findings by hematoxylin and eosin staining in mice. Scale bar=100 μm. Color images available online at www.liebertpub.com/jmf
Effects of CSE on cytokine expression
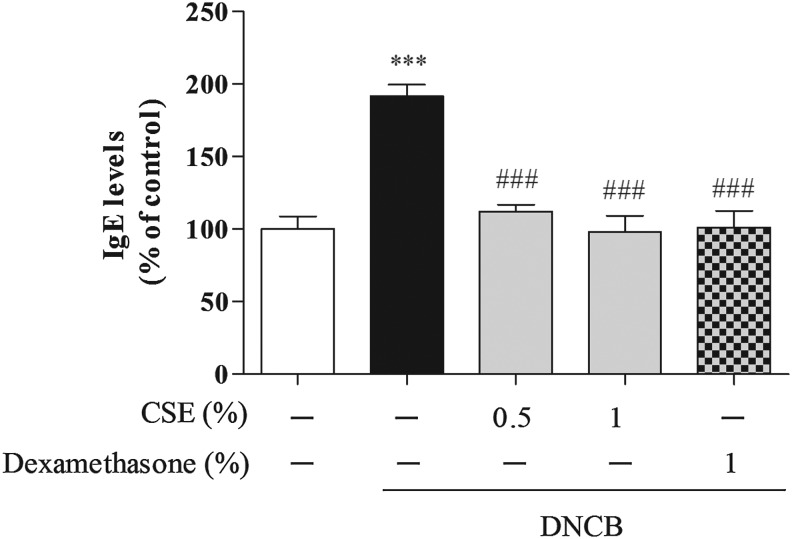
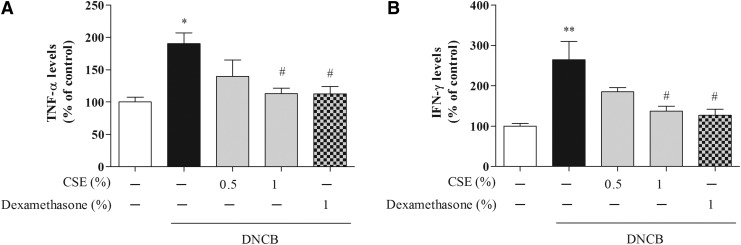
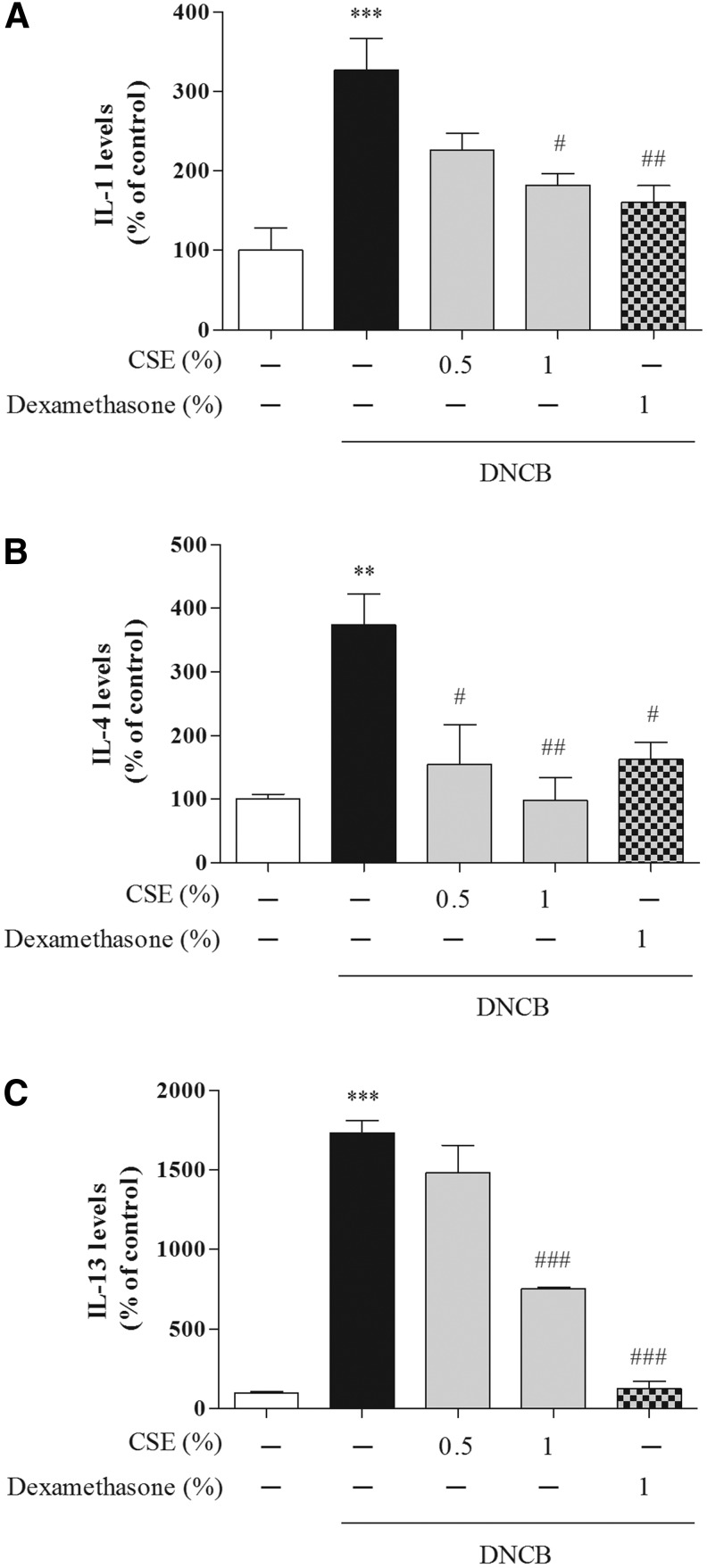
To determine whether CSE affected CD-related cytokines, we determined the serum levels of IgE, TNF-α, IFN-γ, IL-1, IL-4, and IL-13. Treatment with DNCB significantly increased the levels of IgE, TNF-α, and IFN-γ compared with control cells (by 191.69%±7.80%, 147%±12.49%, and 264.54%±45.22%, respectively), whereas treatment with 0.5% and 1% CSE or 1% dexamethasone reduced DNCB-induced IgE (by 112.23%±4.55%–97.92%±11.37% and 101.17%±11.37%, respectively), TNF-α (by 116.10%±21.17%–99.57%±8.48% and 99.33%±11.91%, respectively), and IFN-γ (by 185.27%±10.15%–137.06%±12.59% and 127.30%±14.63%, respectively) (Figs. 4 and 5). Moreover, treatment with DNCB significantly increased the levels of IL-1, IL-4, and IL-13 compared with control cells (by 327.18%±39.44%, 374.54%±47.99%, and 1733.23%±76.97%, respectively), whereas treatment with 0.5% and 1% CSE or 1% dexamethasone reduced DNCB-induced IL-1 (by 192.49%±15.01%–182.10%±14.71% and 160.36%±21.19%, respectively), IL-4 (by 154.68%±61.87%–98.57%±34.81% and 162.48%±27.22%, respectively), and IL-13 (by 1481.26%±172.32%–753.75%±8.03% and 124.56%±47.87%, respectively) (Fig. 6).
FIG. 4.
The effect of CSE on DNCB-induced serum immunoglobulin E (IgE) levels in mice. Values are means±standard error of the mean. ***P<.001 compared with the control group; ###P<.001 compared with the DNCB-alone group.
FIG. 5.
The effect of CSE on DNCB-induced serum levels of tumor necrosis factor (TNF)-α (A) and interferon (IFN)-γ (B) in mice. Values are means±standard error of the mean. *P<.05 and **P<.01 compared with the control group; #P<.05 compared with the DNCB-alone group.
FIG. 6.
The effect of CSE on DNCB-induced serum levels of interleukin (IL)-1 (A), IL-4 (B), and IL-13 (C) in mice. Values are means±standard error of the mean. **P<.01 and ***P<.001 compared with the control group; #P<.05, ##P<.01, and ###P<.001 compared with the DNCB-alone group.
Effects of CSE on oxidative defense enzyme activities such as HO-1 and GSH proteins
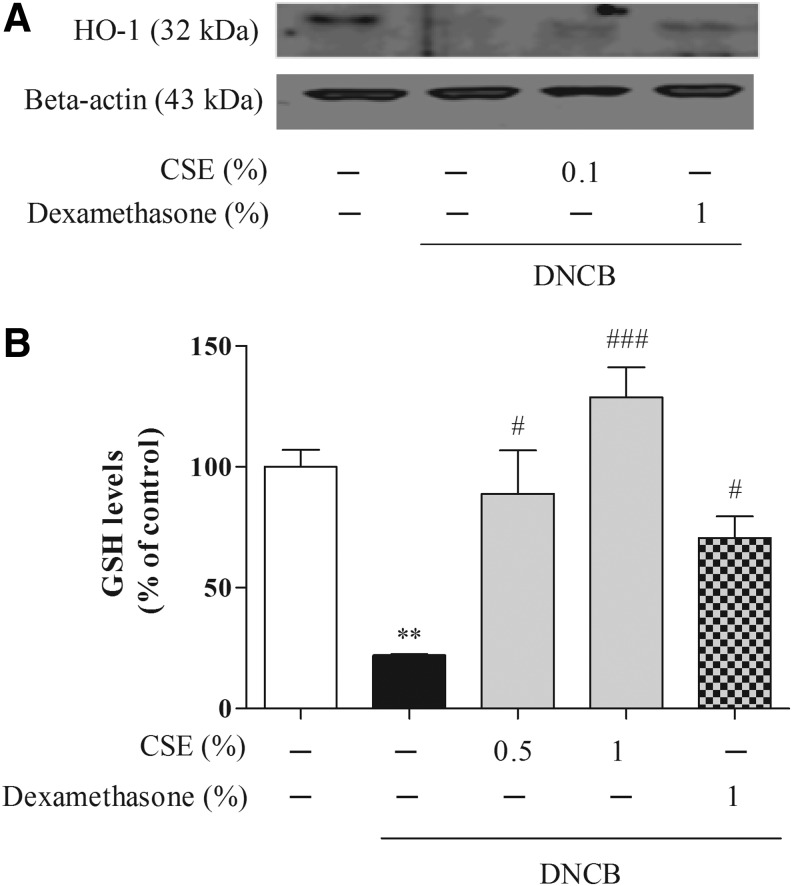
To examine whether CSE affected the oxidative defense enzyme, we measured the levels of antioxidative-related enzyme such as HO-1 and total GSH levels. DNCB caused significant HO-1 and GSH (by 22.05%±0.30%) depletion, whereas CSE at 0.5% or 1% μM increased HO-1 and GSH (by 88.94%±15.58%–128.86%±10.81%) levels (Fig. 7).
FIG. 7.
The effect of CSE on DNCB-induced levels of oxidative regulatory factors such as heme oxygenase-1 (HO-1) (A) and glutathione (GSH) (B) proteins in the skin of mice. Values are means±standard error of the mean. **P<.01 compared with the control group; #P<.05 and ###P<.001 compared with the DNCB-alone group.
Discussion
We demonstrated the protective effect of CSE on DNCB-induced CD-like skin lesions in mice using clinical skin severity scores and scratching behavior, through its regulation of inflammatory mediators: serum IgE, TNF-α, IFN-γ, IL-1, IL-4, and IL-13 levels.
Scratching is a major clinical symptom in skin dermatitis and complicates the management of this pathological condition.13,14 Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs and, like other steroidal drugs, it is commonly prescribed for the alleviation of the symptoms of skin dermatitis.15,16 It is well known that CD skin lesions are characterized by thickened plaques with increased lichenification and fibrotic papules. Treatment with DNCB significantly increased the clinical skin severity scores, scratching scores, and skin thickness compared with the control group, but treatment with 0.5% CSE, 1% CSE, or 1% dexamethasone reduced the DNCB-induced clinical skin severity scores (Fig. 1), scratching scores (Fig. 2), and skin thickness (Fig. 3). These results indicate that CSE inhibits CD-like skin lesions and scratching behavior.
To determine whether CSE affected CD-related cytokines, we determined the serum levels of IgE, TNF-α, IFN-γ, IL-1, IL-4, and IL-13. Cytokines representative of Th2 responses, including IL-4, IL-5, IL-10, and IL-13, are known to play important roles in CD.17 Furthermore, it was reported that Th1-mediated cytokines, such as IL-12 and IFN-γ, produced by Th1 cells acted as antagonists for these Th2 cytokines.18,19 In the immediate allergic reaction phase, IgE produced, which is bound to the mast cell through the Fcɛ receptor.20 Crosslinking of allergen-specific IgE leads to the release of various chemical mediators from mast cells.20 The release of these chemical mediators results in various reactions, including itching in the tissue, after a few minutes.21 In the late phase of the allergic reaction, IL-4, IL-13, and other mediators, including chemokines and enzymes are produced and released. Chemokines attract eosinophils to the CD lesion.21 We demonstrated that DNCB significantly increased the levels of IgE, TNF-α, and IFN-γ compared with the control group, whereas treatment with 0.5% CSE, 1% CSE, or 1% dexamethasone reduced DNCB-induced IgE, TNF-α, and IFN-γ (Figs. 4 and 5). Moreover, DNCB significantly increased the serum levels of IL-1, IL-4, and IL-13 compared with the control group, whereas treatment with 0.5% CSE, 1% CSE, or 1% dexamethasone reduced DNCB-induced IL-1, IL-4, and IL-13 (Fig. 6). These results suggest that CSE inhibits Th1- and Th2-mediated cytokines, including IgE, TNF-α, IFN-γ, IL-1, IL-4, and IL-13.
In this study, we demonstrated for the first time that CSE has a protective effect against DNCB-induced CD-like skin lesions. We reported previously that CSE contained 47.06 mg/g fatty acids using gas chromatography analysis.12 Especially, linoleic acid and linolenic acid were the major fatty acids identified, which have been reported to have anti-inflammatory and antioxidant effects in previous studies (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jmf).12,22–24 According to the US National Institutes of Health, National Library of Medicine, there is good scientific evidence for the efficacy of γ-linolenic acid-rich evening primrose oil in the treatment of eczema (atopic dermatitis) and skin irritation.23 Additionally, fatty acid is well known to have free radical scavenging effects.24 Furthermore, CSE increased oxidative defense enzymes in skin (Fig. 7). Therefore, the linoleic acid and linolenic acid in CSE may contribute to the observed protection by CSE of skin from DNCB-induced CD-like skin lesions. However, according to the US Food and Drug Administration, allergic reactions by small amount of compounds in CS are possible in susceptible persons.25 Further detailed studies on the compounds and individual side effects in CS are needed.
In conclusion, our study demonstrates that CS inhibits DNCB-induced CD-like skin lesions, which are mediated by the regulation of Th1- and Th2-mediated cytokines and oxidative defense enzymes.
Supplementary Material
Acknowledgments
This work was supported by the Bio-Synergy Research Project (NRF-2012M3A9C4048795) of the Ministry of Science, ICT, and Future Planning through the National Research Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nielsen NH, Linneberg A, Menne T, Madsen F, Frolund L, Dirksen A, Jørgensen T: Allergic contact sensitization in an adult Danish population: two cross-sectional surveys eight years apart (the Copenhagen Allergy Study). Acta Derm Venereol 2001;81:31–34 [DOI] [PubMed] [Google Scholar]
- 2.Liu F-T, Goodarzi H, Chen H-Y: IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol 2011;41:298–310 [DOI] [PubMed] [Google Scholar]
- 3.Farage MA: Vulvar susceptibility to contact irritants and allergens: a review. Arch Gynecol Obstet 2005;272:167–172 [DOI] [PubMed] [Google Scholar]
- 4.Nermes M, Kantele J, Atosuo T, Salminen S, Isolauri E: Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin Exp Allergy 2011;41:370–377 [DOI] [PubMed] [Google Scholar]
- 5.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG: Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71–109 [DOI] [PubMed] [Google Scholar]
- 6.Kim C-H, Park CD, Lee A-Y: Administration of poly (I: C) improved dermatophagoides farinae-induced atopic dermatitis-like skin lesions in NC/Nga mice by the regulation of Th1/Th2 balance. Vaccine 2011;30:2405–2410 [DOI] [PubMed] [Google Scholar]
- 7.Kosaka S, Tamauchi H, Terashima M, Maruyama H, Habu S, Kitasato H: IL-10 controls Th2-type cytokine production and eosinophil infiltration in a mouse model of allergic airway inflammation. Immunobiology 2011;216:811–820 [DOI] [PubMed] [Google Scholar]
- 8.Fabricius D, Neubauer M, Mandel B, Schütz C, Viardot A, Vollmer A, Jahrsdörfer B, Debatin KM: Prostaglandin E2 inhibits IFN-α secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J Immunology 2010;184:677–684 [DOI] [PubMed] [Google Scholar]
- 9.Sahib NG, Anwar F, Gilani AH, Hamid AA, Saari N, Alkharfy KM: Coriander (Coriandrum sativum L.): A Potential source of high-value components for functional foods and nutraceuticals—a review. Phytother Res 2013;27:1439–1456 [DOI] [PubMed] [Google Scholar]
- 10.Wu TT, Tsai CW, Yao HT, Lii CK, Chen HW, Wu YL, Chen PY, Liu KL: Suppressive effects of extracts from the aerial part of Coriandrum sativum L. on LPS-induced inflammatory responses in murine RAW 264.7 macrophages. J Sci Food Agric 2010;90:1846–1854 [DOI] [PubMed] [Google Scholar]
- 11.Reuter J, Huyke C, Casetti F, Theek C, Frank U, Augustin M, Schempp C: Anti-inflammatory potential of a lipolotion containing coriander oil in the ultraviolet erythema test. J Dtsch Dermatol Ges 2008;6:847–851 [DOI] [PubMed] [Google Scholar]
- 12.Park G, Kim H, Kim Y, Park S, Kim S, Oh M: Coriandrum sativum L. protects human keratinocytes from oxidative stress by regulating oxidative defense systems. Skin Pharmacol Physiol 2012;25:93–99 [DOI] [PubMed] [Google Scholar]
- 13.Katayama I, Kohno Y, Akiyama K, Ikezawa Z, Kondo N, Tamaki K, Kouro O: Japanese guideline for atopic dermatitis. Allergol Int 2011;60:205. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim SF, Benedetto AD, Beck LA: Update on the management of atopic dermatitis/eczema. allergy frontiers. Ther Prev 2010;5:259–290 [Google Scholar]
- 15.He Q, Huang H-Y, Zhang Y-Y, Li X, Qian S-W, Tang Q-Q: TAZ is downregulated by dexamethasone during the differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res Commun 2012;419:573–577 [DOI] [PubMed] [Google Scholar]
- 16.Samukawa K, Izumi Y, Shiota M, Nakao T, Osada-Oka M, Miura K, Iwao H: Red ginseng inhibits scratching behavior associated with atopic dermatitis in experimental animal models. J Pharmacol Sci 2012;118:391–400 [DOI] [PubMed] [Google Scholar]
- 17.Yamaura K, Suwa E, Ueno K: Repeated application of glucocorticoids exacerbate pruritus via inhibition of prostaglandin D2 production of mast cells in a murine model of allergic contact dermatitis. J Toxicol Sci 2011;37:1127–1134 [DOI] [PubMed] [Google Scholar]
- 18.Yamashita H, Ito T, Kato H, Asai S, Tanaka H, Nagai H, Inagaki N: Comparison of the efficacy of tacrolimus and cyclosporine A in a murine model of dinitrofluorobenzene-induced atopic dermatitis. Eur J Pharmacol 2010;645:171–176 [DOI] [PubMed] [Google Scholar]
- 19.Byrne AM, Goleva E, Chouiali F, Kaplan MH, Hamid QA, Leung DY: Induction of GITRL expression in human keratinocytes by Th2 cytokines and TNF-α: implications for atopic dermatitis. Clin Exp Allergy 2012;42:550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, Fujiwara S: The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol 2013;22:30–35 [DOI] [PubMed] [Google Scholar]
- 21.Raap U, Weißmantel S, Gehring M, Eisenberg AM, Kapp A, Fölster-Holst R: IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol 2012;23:285–288 [DOI] [PubMed] [Google Scholar]
- 22.Thijs C, Müller A, Rist L, Kummeling I, Snijders B, Huber M, van Ree R, Simões-Wüst AP, Dagnelie PC, van den Brandt PA: Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy 2010;66:58–67 [DOI] [PubMed] [Google Scholar]
- 23.Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S: Probiotics in the management of atopic eczema. Clin Exp Allergy 2008;30:1605–1610 [DOI] [PubMed] [Google Scholar]
- 24.Kalmijn S, Feskens EJM, Launer LJ, Kromhout D: Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol 1997;145:33–41 [DOI] [PubMed] [Google Scholar]
- 25.Burdock GA, Carabin IG: Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem Toxicol 2009;47:22–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.