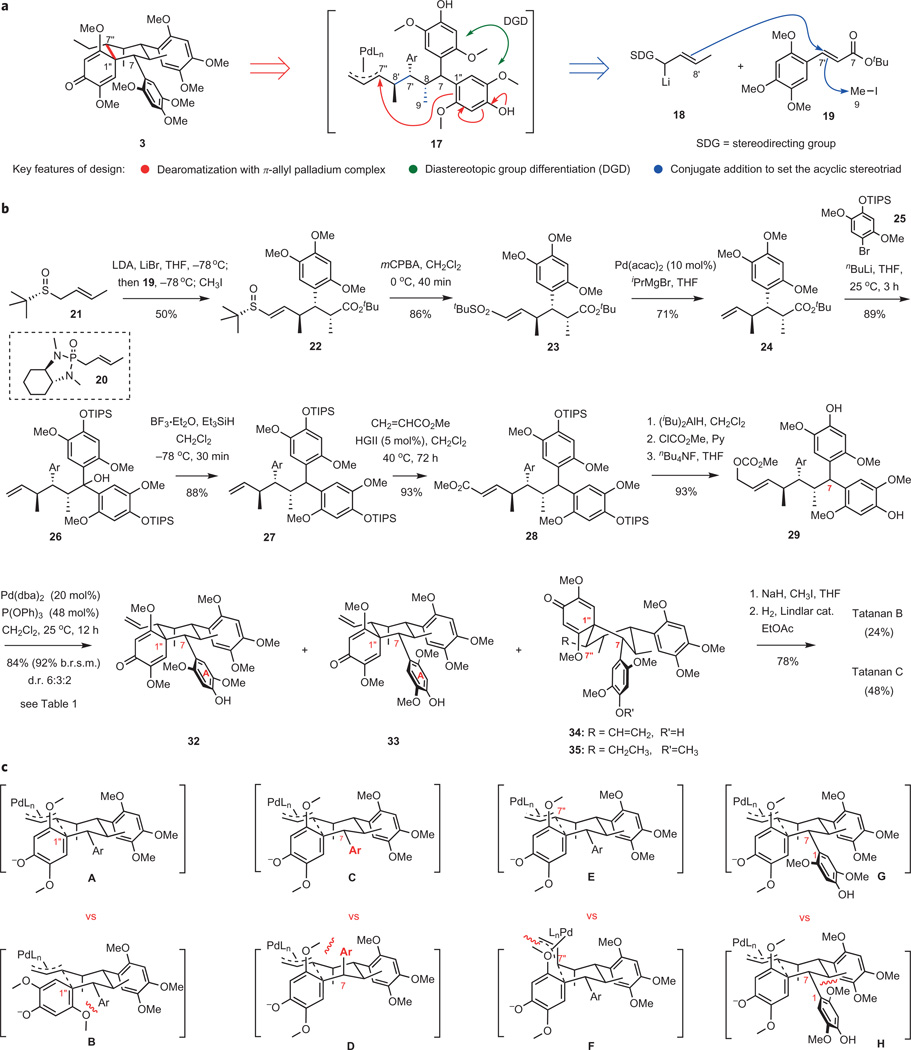
Figure 4. Total synthesis of tatanans B and C by a palladium-catalysed cyclodearomatization.
a, Reformulated synthesis plan centred on a convergent strategy enabled by a stereochemically complex palladium-catalysed cyclization featuring diastereotopic aromatic group differentiation with concomitant construction of the central quaternary centre. b, Successful implementation of the convergent synthesis plan leading to a separable mixture of atropisomeric tatanans B and C in 12 steps from sulfoxide 21. c, Four pairs of transition structures rationalizing all four stereogenic events in the complex palladium-catalysed cyclodearomatization of substrate 29. Structures A and B explain the observed selectivity for the formation of the C1″ quaternary centre. Structures C and D provide the basis for the observed high selectivity during the diastereotopic group differentiation establishing stereochemistry at C7. Structures E and F explain the preference for the observed selectivity at C7″, favouring the vinyl substituent at the equatorial position. Structures G and H rationalize the observed atropselectivity, with a moderate preference for G over H, which places the ortho-MeO group in ring A in a sterically crowded environment of the forming cyclohexane ring. LDA, lithium diisopropylamide; MCPBA, 3-chloroperbenzoic acid; acac, acetylacetonate; TIPS, triisopropylsilyl; HGII, Hoveyda– Grubbs II catalyst; dba, dibenzylideneacetone.