Abstract
Background
Resistance exercise effects on cardiovascular parameters are not consistent.
Objectives
The effects of resistance exercise on changes in blood glucose, blood pressure and vascular reactivity were evaluated in diabetic rats.
Methods
Wistar rats were divided into three groups: control group (n = 8); sedentary diabetic (n = 8); and trained diabetic (n = 8). Resistance exercise was carried out in a squat device for rats and consisted of three sets of ten repetitions with an intensity of 50%, three times per week, for eight weeks. Changes in vascular reactivity were evaluated in superior mesenteric artery rings.
Results
A significant reduction in the maximum response of acetylcholine-induced relaxation was observed in the sedentary diabetic group (78.1 ± 2%) and an increase in the trained diabetic group (95 ± 3%) without changing potency. In the presence of NG-nitro-L-arginine methyl ester, the acetylcholine-induced relaxation was significantly reduced in the control and trained diabetic groups, but not in the sedentary diabetic group. Furthermore, a significant increase (p < 0.05) in mean arterial blood pressure was observed in the sedentary diabetic group (104.9 ± 5 to 126.7 ± 5 mmHg) as compared to that in the control group. However, the trained diabetic group showed a significant decrease (p < 0.05) in the mean arterial blood pressure levels (126.7 ± 5 to 105.1 ± 4 mmHg) as compared to the sedentary diabetic group.
Conclusions
Resistance exercise could restore endothelial function and prevent an increase in arterial blood pressure in type 1 diabetic rats.
Keywords: Rats; Exercise; Physical endurance; Endothelium, vascular / physiology; Arterial Pressure / physiology; Diabetes
Introduction
Diabetes mellitus is a heterogeneous group of metabolic disorders that have in common hyperglycemia associated with secondary cardiovascular system complications1,2. Increased blood glucose levels are associated with in vivo and in vitro endothelial dysfunction3,4. Endothelial dysfunction is a systemic phenomenon related to an unbalance in the endothelial production of mediators that regulate vascular tone; it contributes partially to increase arterial blood pressure levels5. The endothelial dysfunction in type 1 diabetes mellitus can be considered an early marker of cardiovascular disease6.
Several factors, such as hyperlipidemia, insulin resistance, hyperglycemia and hypertension, can explain the endothelial dysfunction in type 1 diabetes mellitus7. Resistance exercise has been reported to contribute to prevent/treat pathologies that affect the metabolism and cardiovascular function8-10. Resistance exercise has proved to have an important therapeutic potential by promoting skeletal muscle mass gain, increased insulin sensitivity and blood glucose reduction in diabetic rats8,11. Aerobic exercise also have those effects11,12.
Some studies have suggested that aerobic exercise is effective to treat endothelial dysfunction in diabetes13-15. However, little is known about the chronic effects of resistance exercise on the arterial blood pressure and endothelial function of type 1 diabetic rats. We raised the hypothesis that long-term resistance exercise can minimize the deleterious effects on the cardiovascular system and on the metabolic control of type 1 diabetes mellitus-induced animals. Thus, this study aimed at assessing the chronic effects of resistance exercise on blood glucose changes, vascular reactivity and arterial blood pressure of diabetic rats.
Methods
Animals and experimental delineation
Male Wistar rats (Rattus norvegicus), aged 3 months, weighing 250-300 g, were used in all experiments. They were kept under controlled temperature (22 ± 1 °C) and 12-hour light-dark cycles, with free access to water and food specific for rodents (Labina, Purina®). All procedures described in this study were approved by the Committee on Ethics and Animal Research of the Universidade Federal de Sergipe (UFS) (protocol number 01/2008). The animals were divided into the following three groups of eight animals each: control group (C); diabetic sedentary (DS); and diabetic trained (DT). In groups C and DS, the animals were kept in their cages and not exposed to exercise, while group DT animals underwent eight weeks of resistance exercise.
Drugs
The following drugs were used: acetylcholine chloride (ACh); L-phenylephrine (Phe); NG-nitro-L-arginine methyl ester (L-NAME); alloxan (SIGMA®, USA); and sodium thiopental (Thiopentax, Cristália, Itapira, SP, Brazil).
Induction of diabetes and blood glucose measurement
Experimental diabetes was induced as described by Da Silva Costa et al16. After a 24-hour fasting period, diabetes was induced by use of a single intravenous dose of alloxan (40 mg/kg - penile vein), two weeks before starting the exercise protocol. The animals with blood glucose level ≥ 200 mg/dL were selected as diabetic. Blood glucose level was measured one week after alloxan administration by using reagent test strips (ACCU-CHEK Advantage II, Roche®, São Paulo, SP, Brazil) coupled to a portable digital glucometer (ACCU-CHEK Advantage II, Roche®, São Paulo, SP, Brazil).
Exercise protocol
Resistance exercise was performed in a squat device according to the model by Tamaki et al17. After one week of adaptation, DT animals underwent training with three sets of ten repetitions, with 60-second rest intervals and intensity of 50% of the load established through the one-repetition maximum (1RM) test, three times a week. For determining the maximum strength, successive loads were added to the equipment, and the animals were electrically stimulated to repeat the exercise. Between load increments, 5-minutes rests were observed to allow the musculature to recover. The maximum load for each animal was the maximum amount of weight allowing the complete movement. Training loads were readjusted every two weeks by using a new 1RM test18. The parameters of electrical stimulation were those described by Cássia Cypriano Ervati Pinter et al19. Electrical stimulation (20 V, 0.3-second duration, 3-second interval)20 with electrodes (Valu Trode, Model CF3200, Axelgaard, Fallbrook, CA, USA) fixed to the animals' tails and connected to an electrical stimulator (BIOSET, Physiotonus Four, Model 3050, Rio Claro, SP, Brazil) was used to make the animals performed their exercise sets.
Surgical procedure and direct recording of mean arterial blood pressure
For this procedure, the animals were anesthetized with sodium thiopental (45 mg/kg, intraperitoneal), and a polyethylene catheter (PE-10/50, Intramedic, Becton Dickinson and Company, Sparks, MD, USA) with heparin saline (1:20 v/v) was implanted through an inguinal incision in the left femoral artery to record arterial blood pressure. After insertion and fixation, the catheter was exteriorized in the animal's posterior cervical region (scapulae) and the incision, sutured. After finishing the surgical procedures, all animals received a single intramuscular dose (0.2 g/kg) of oxytetracycline hydrochloride (long-lasting antibiotic) and oral sodium diclofenac (10 mg/kg/day). Then, the animals were put into individual cages, where they remained for a minimum period of 24 hours (postoperative recovery).
After the animals recovered and were moving spontaneously, the catheter was coupled to a pressure transducer (Edwards Lifescience®, Irvine, CA, USA), and 30 minutes after the signal stabilized, a 5-minute recording was performed. After recording the mean arterial blood pressure for 24 hours, the animals were anesthetized and prepared for the vascular reactivity experiments.
Vascular reactivity of the superior mesenteric artery
The tissue was prepared as described by Araujo et al10. Endothelial function was assessed via the ability of 1 µM of ACh to induce relaxation in more than 75% of the previously contracted superior mesenteric artery rings with 1 µM of FEN21.
The vascular reactivity changes were assessed by using concentration-response curves for ACh (10-9-10-4 M), a non-selective muscarinic agonist. To assess the nitric oxide (NO) participation in ACh-induced relaxation, the curves for that agent were also obtained in the presence of L-NAME (100 µM), a nitric oxide synthase (NOS) inhibitor.
Statistical analyses
Initially, all data underwent the Kolmogorov-Smirnov test to determine whether the probability distributions were parametric or non-parametric. All data had normal distribution. The values were expressed as mean ± standard error of the mean (SEM). When necessary, Student t test for independent samples and repeated measures or two-way analysis of variance (ANOVA), followed by the Bonferroni post-test, were used to assess the significance of the differences between the means. Pearson's correlation was used to determine the association between ACh-induced relaxation and blood glucose levels. The significance level adopted was p < 0.05. The GraphPad Prism statistical software, version 3.02 (GraphPad Software, San Diego, CA, USA), was used for all procedures.
Results
Maximum strength
At the beginning of the experiment, the strength levels were similar in all groups (C: 956.3 ± 63.3 g, n = 8; DS: 1,022.2 ± 32.3 g, n = 8; and DT: 945.4 ± 108.7 g, n=8). After eight weeks, the strength levels of animals in groups C and DS showed no statistically significant difference (1,032.2 ± 44.0 and 1,030.5 ± 61.2 g, respectively). In addition, the resistance exercise promoted an increase in strength levels from 945.4 ± 108.7 g to 1,327.3 ± 98.7 g (p < 0.01).
Blood glucose levels
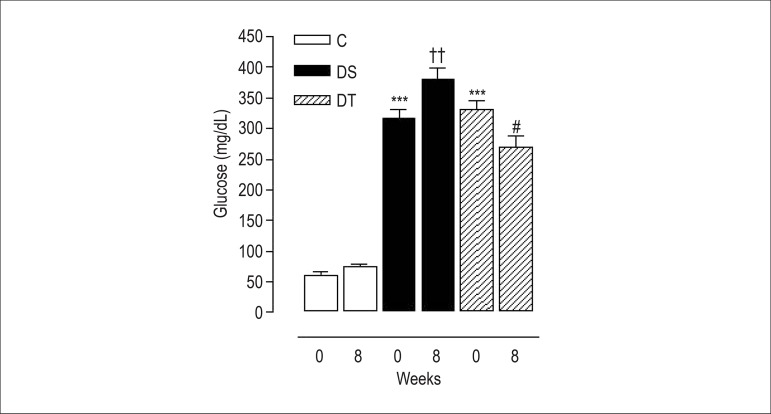
Figure 1 shows the effect of resistance exercise on blood glucose levels. Alloxan induced an increase (p < 0.001) in blood glucose concentration in both experimental groups. In addition, resistance exercise caused a reduction (p < 0.05) in blood glucose levels after eight weeks (Figure 1).
Figure 1.
Variation of blood glucose levels of rats at the beginning (0) and by the end (8) of eight weeks of training: control group (C); diabetic sedentary group (DS); and diabetic trained group (DT). Data are expressed as mean ± standard error of the mean. The statistical differences were determined by repeated measures analysis of variance followed by Bonferroni post-test. *** p < 0.001 vs. C 0; †† p < 0.01 vs. DS 0; and # p < 0.05 vs. DT 0.
Endothelium-dependent relaxation
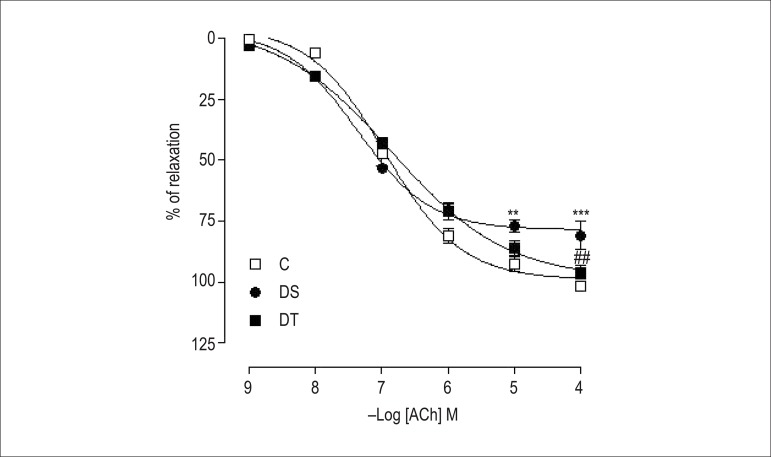
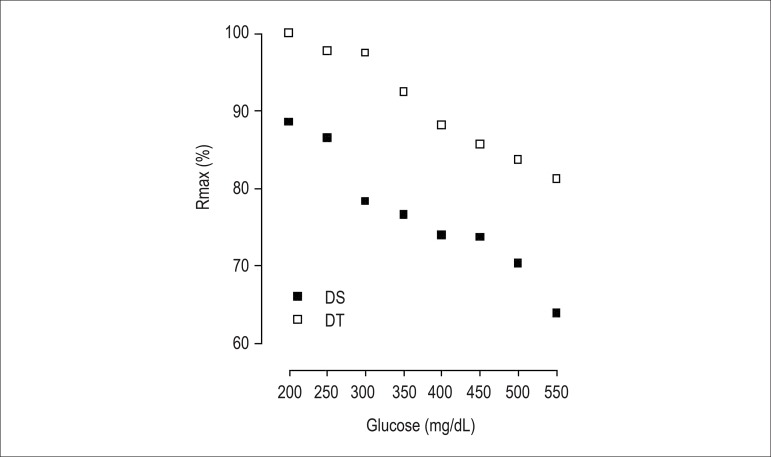
Figure 2 shows ACh induced relaxation, depending on its concentration, in isolated rings of the superior mesenteric artery, with intact endothelium in all groups. Neither diabetes, nor resistance exercise interfered with arterial sensitivity, considering that the concentration from which the agonist produces 50% of maximum response (potency - pD2) remained unaltered. However, in group DS, diabetes reduced (p < 0.001) the maximum response (Rmax) as compared to that in group C. That was reversed (p < 0.01) in DT animals. In addition, a strong negative correlation was observed between ACh-induced relaxation and blood glucose levels in groups DS (r = -0.9710; p = 0.001, n = 8) and DT (r = -0.9874; p = 0.001, n = 8) (Figure 3).
Figure 2.
Concentration-response curves for acetylcholine (ACh: 10-9 – 10 -4 M) in isolated superior mesenteric artery rings, with intact endothelium and pre-contracted with L-phenylephrine (1 μM). The rings were obtained from rats of the groups control (C), diabetic sedentary (DS) and diabetic trained (DT). Data are expressed as mean ± standard error of the mean. The statistical differences were determined by two-way analysis of variance followed by Bonferroni post-test. ** p < 0.01 and *** p < 0.001 vs. C; ## p < 0.01 vs. DS.
Figure 3.
Correlation between blood glucose levels and the percentage of maximum response of acetylcholine-induced relaxations in superior mesenteric artery rings of the groups diabetic sedentary (A) and diabetic trained (B).
Endothelium-dependent relaxation in the presence of L-NAME
Table 1 shows that L-NAME could reduce (p < 0.001) pD2 and Rmax (p < 0.01 and p < 0.001) of ACh-induced relaxations in groups C and DT, respectively. That reduction was not observed in group DS. The presence of L-NAME significantly increased (p < 0.001) the pD2 of ACh-induced relaxations of group DS as compared to group C, without modifying the Rmax. However, L-NAME significantly reduced (p < 0.001) the pD2 and Rmax (p < 0.01) of the ACh-induced relaxations of group DT as compared to group DS.
Table 1.
Potency (pD2) and maximum response (Rmax) obtained from concentration-response curves for acetylcholine before and after pretreatment with NG-nitro-L-arginine methyl ester (L-NAME)
| Groups | Condition | pD2 | Rmax |
|---|---|---|---|
| C | - L-NAME | 7.0 ± 0.0 | 99.0 ± 2.7 |
| + L-NAME | 5.5 ± 0.1* | 70.0 ± 6.2** | |
| DS | - L-NAME | 7.2 ± 0.0 | 78.0 ± 1.8 |
| + L-NAME | 6.8 ± 0.1† | 72.4 ± 3.1 | |
| DT | - L-NAME | 7.0 ± 0.1 | 95.0 ± 3.5 |
| + L-NAME | 4.9 ± 0.2* # | 50.0 ± 3.6* ## |
The superior mesenteric artery rings were obtained from rats of the groups control (C), diabetic sedentary (DS), and diabetic trained (DT). The experiments were performed in the absence of L-NAME (- L-NAME) or presence of 100 μM of L-NAME (+ L-NAME). Data are expressed as mean ± standard error of the mean. The statistical differences were determined by Student t test for independent samples (intragroup) or analysis of variance followed by Bonferroni post-test (intergroup).
* p <0.001 or **p <0.01 for - L-NAME vs. + L-NAME values; † p<0.001 vs. C and # p<0.001 or ## p<0.01 vs. DS.
Mean arterial blood pressure
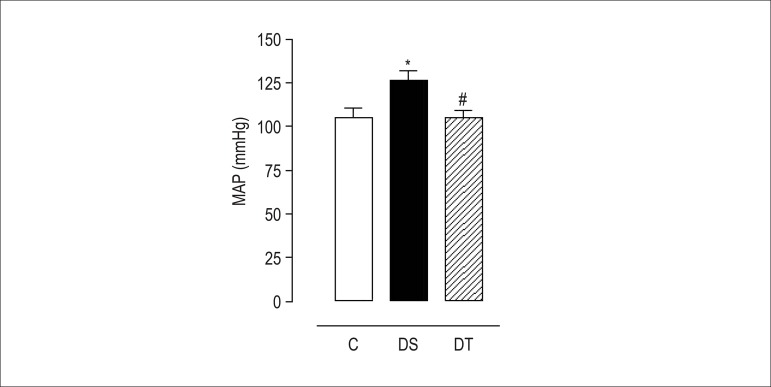
Diabetes induction with alloxan increased (p < 0.05) mean arterial blood pressure in group DS. Inversely, resistance exercise reduced (p < 0.05) arterial blood pressure levels of group DT animals (Figure 4).
Figure 4.
Mean arterial blood pressure of rats of the groups control (C), diabetic sedentary (DS), and diabetic trained (DT) after eight weeks of resistance exercise. Data are expressed as mean ± standard error of the mean. The statistical differences were determined by repeated measures analysis of variance followed by Bonferroni post-test. * p < 0.05 DS vs. C and # p < 0.05 DT vs. DS.
Discussion
According to the results, resistance exercise reduces blood glucose levels, restores endothelial function and decreases arterial blood pressure of type 1 diabetic animals after eight weeks of training.
Resistance exercise is characterized by intermittent movements and a predominantly anaerobic metabolic pathway22,23. In this study, resistance exercise was performed in a squat device for rats, and proved to be effective in mimicking the beneficial cardiovascular effects found in humans practicing that type of exercise19,20. Tail electrical stimulation was required for the animals to perform the squat movement. The electrical stimulation parameters used in this study have been reported to promote no cardiovascular system changes20. Based on the literature, we suggest that the effects observed in trained diabetic animals are directly related to resistance exercise.
The maximum strength test was used as an indicator of training efficacy. From that perspective, we observed that trained diabetic animals gained muscle strength after eight weeks. This indicates that the training protocol could promote chronic adjustments resulting from resistance exercise. The importance of the therapeutic potential of that type of exercise has been recently emphasized9. Resistance exercise has proved to have a beneficial effect on insulin action improvement, muscle mass gain, fatty mass reduction, blood glucose control and arterial blood pressure reduction in individuals with diabetes24-26.
Experimental and clinical evidence has shown that metabolic disorders, mainly the chronic increase in blood glucose levels, are strictly related to cardiovascular complications of diabetes mellitus7,26. To better understand the metabolic and cardiovascular complications originating from diabetes, several experimental models of diabetes induced in rats have been widely used by different research groups27. Alloxan has been reported to cause the destruction of a large number of beta-pancreatic cells, hindering insulin production27,28. The experimental model of alloxan-induced diabetes is of type 1, with symptoms similar to those found in humans, such as weight loss, polyuria, polydipsia, polyphagia, glycosuria, ketonuria, increased production of oxygen reactive species, decreased blood insulin levels and increased blood glucose levels27-29.
The increased blood glucose levels of diabetic animals observed at the beginning of the study were reduced after eight weeks of training. Similarly, Farrell et al8 have shown that treatment with resistance exercise, by the end of eight weeks, reduced blood glucose levels of animals with type 1 diabetes. Studies have shown that muscle contraction during physical exercise stimulates the translocation of glucose transporter type 4 (GLUT4), independently of insulin action, resulting in an increase in peripheral glucose uptake30,31. Thus, a possible explanation for blood glucose level reduction in trained animals in this study could relate to a greater activation in the signaling pathways involved in glucose transportation regardless of insulin action, because our animals had insulin deficiency or lack of insulin production, since ours was a type 1 diabetes mellitus model8,28,32.
According to Gross et al33, increased blood glucose levels cause damages, dysfunctions and even failure of several organs, involving severe micro- and macrovascular changes. In some cases, restoration of normal blood glucose levels reverts cell damages. In others, however, such damages are irreversible, making blood glucose control a physiological parameter of essential importance to prevent the severe chronic complications of diabetes30-33. Studies have shown that diabetes mellitus causes changes in endothelium-dependent relaxation of different vascular beds, promoting endothelial dysfunction34,35. Endothelial dysfunction is considered a biomarker of cardiovascular risk, and the importance of the endothelium in maintaining vascular health is a consensus in the literature36.
The group DS animals showed loss of vascular function. However, eight weeks of resistance exercise could restore the vascular function of diabetic animals. That can be due to the reduction in blood glucose levels observed in group DT animals. Our results evidenced that the ACh-induced relaxation had a strong inverse correlation with blood glucose levels. The group DS animals had an increase in blood glucose levels and an important loss in endothelial function, however, the reduction in blood glucose levels is associated with the vascular function restoration of group DT animals. Such results emphasize the findings of several other studies, which indicate resistance exercise as a possible tool to treat and/or prevent diseases with vascular function loss, such as hypertension and diabetes8,10,19,20,38.
Diabetes mellitus has been reported to reduce the endothelial production of vasoactive substances responsible for regulating the vascular tone, such as NO and prostaglandins1. To investigate the participation of NO in endothelium-dependent relaxations, concentration-response curves for ACh were obtained in the presence of L-NAME. Under that experimental condition, L-NAME antagonized the ACh-induced relaxation in animals of groups C and DT, but did not change the relaxation in group DS animals, characterizing a reduction in the participation of one of the major endothelium-derived relaxing factors in group DS animals. Those findings are in accordance with the results by Chen et al39, who have reported that ACh-induced Rmax was also reduced in the presence of L-NAME in animals undergoing aerobic exercise for eight weeks.
Group DT animals had a higher percentage of relaxation inhibition in the presence of L-NAME as compared with healthy animals. That phenomenon might have resulted from a possible increase in NO-dependent relaxation caused by resistance exercise. The increase in relaxations observed in this study corroborates other findings, in which there was an increase in aerobic-exercise-mediated NO production in the experimental type 1 diabetes model14.
Studies with humans with type 1 diabetes have also shown that aerobic exercise improved endothelial function in vascular beds not directly involved during exercise13. In this study, the effects observed in the arteries of animals exercising also suggest a possible generalized vascular effect, because the artery analyzed was far from the tissues more active during exercise. That generalized vascular effect has also been observed by Faria et al38, and one single session of resistance exercise increased the NO-dependent relaxation in the caudal artery, reducing arterial blood pressure in spontaneously hypertensive rats.
In addition, in our study, resistance exercise reduced mean arterial blood pressure in DT animals, proving to be effective to treat endothelial dysfunction related to increased blood glucose levels. Recent data from our laboratory have shown that L-NAME-induced hypertensive animals also showed a reduction in arterial blood pressure levels after four weeks of resistance exercise10. The mechanisms responsible for the decrease in arterial blood pressure of animals undergoing resistance exercise can be the reduction in peripheral vascular resistance and the increase in systemic vascular conductance39.
Thus, our results suggest that low-intensity resistance exercise induces metabolic and cardiovascular responses similar to those observed in studies submitting diabetic animals to aerobic exercise14,15. Although those modalities of exercise have different characteristics, such as the energetic pathway and movement execution, both promote beneficial cardiometabolic effects that help to treat both types of diabetes mellitus11,12.
Thus, this study indicates that the model of resistance exercise used could reduce blood glucose levels, restore endothelial function and reduce arterial blood pressure in diabetic animals. Finally, resistance exercise might cause beneficial vascular and metabolic adjustments for the treatment of type 1 diabetes mellitus dysfunctions in an experimental model40.
Acknowledgements
We thank the Brazilian Board of Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher-Level-Education Personnel (CAPES) and the Foundation for Support to Technological Research and Innovation of the State of Sergipe (FAPITEC-SE) for the financial support.
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception and design of the research: Mota MM, Silva TLTB, Barreto AS, Santos MRV; Acquisition of data: Mota MM, Silva TLTB, Fontes MT, Araújo JES; Analysis and interpretation of the data: Mota MM, Silva TLTB, Fontes MT, Oliveira ACC; Statistical analysis: Mota MM, Silva TLTB, Barreto AS; Obtaining financing: Santos MRV; Writing of the manuscript: Mota MM, Silva TLTB, Wichi RB; Critical revision of the manuscript for intellectual content: Mota MM, Silva TLTB, Oliveira ACC, Wichi RB, Santos MRV.
Sources of Funding
This study was funded by CNPq, CAPES e FAPITEC/SE.
Study Association
This article is part of the thesis of master submitted by Marcelo Mendonça Mota, from Programa de Pós-Graduação em Ciências da Saúde da Universidade Federal de Sergipe (UFS).
References
- 1.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Singh R, Vasudeva N, Sharma S. Acute and chronic animal models for the evaluation of anti-diabetic agents. Cardiovasc Diabetol. 2012;11:9–9. doi: 10.1186/1475-2840-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87(5):1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97(1):22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang EH, Vanhoutte PM. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Arch. 2010;459(6):995–1004. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- 6.Schram N, Chaturvedi C, Schalkwijk C, Giorgino F, Ebeling P, Fuller JH, et al. Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB prospective complications study. Diabetes Care. 2003;26(7):2165–2173. doi: 10.2337/diacare.26.7.2165. [DOI] [PubMed] [Google Scholar]
- 7.Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes Mde B. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789. doi: 10.1155/2013/653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell PA, Fedele MJ, Hernandez J, Fluckey JD, Miller JL, 3rd, Lang CH, et al. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. J Appl Physiol. 1999;87(3):1075–1082. doi: 10.1152/jappl.1999.87.3.1075. [DOI] [PubMed] [Google Scholar]
- 9.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113(22):2642–2650. doi: 10.1161/CIRCULATIONAHA.105.584060. [DOI] [PubMed] [Google Scholar]
- 10.Araujo AJ, Santos AC, Souza KS, Aires MB, Santana-Filho VJ, Fioretto ET, et al. Resistance training controls arterial blood pressure from L-NAME induced hypertensive rats. Arq Bras Cardiol. 2013;100(4):339–346. [PubMed] [Google Scholar]
- 11.Hall KE, McDonald MW, Grisé KN, Campos OA, Noble EG, Melling CW. The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced Type 1 diabetic rodents. Metabolism. 2013;62(10):1485–1494. doi: 10.1016/j.metabol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Mann S, Beedie C, Balducci S, Zanuso S, Allgrove J, Bertiato F, et al. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab Res Rev. 2013 Oct 15; doi: 10.1002/dmrr.2488. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Fuchsjäger-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25(10):1795–1801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- 14.Chakraphan D, Sridulyakul P, Thipakorn B, Bunnag S, Huxley VH, Patumraj S. Attenuation of endothelial dysfunction by exercise training in STZ-induced diabetic rats. Clin Hemorheol Microcirc. 2005;32(3):217–226. [PubMed] [Google Scholar]
- 15.Mota MM, Silva TL, Fontes MT, Barreto AS, Oliveira AC, Santos MR. Treinamento aeróbio previne alterações na vasodilatação dependente do endotélio em ratos diabéticos. Rev educ fis UEM. 2013;24(3) [Google Scholar]
- 16.da Silva Costa EC, Goncalves AA, Areas MA, Morgabel RG. Os efeitos da metformina sobre a dispersão do intervalo QT e QTc de ratos diabéticos. Arq Bras Cardiol. 2008;90(4):254–260. doi: 10.1590/s0066-782x2008000400004. [DOI] [PubMed] [Google Scholar]
- 17.Tamaki T, Uchiyama S, Nakano S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med Sci Sports Exerc. 1992;24(8):881–886. [PubMed] [Google Scholar]
- 18.Galdino GS, Duarte IDG, Perez AC. Participation of endogenous opioids in the antinociception induced by resistance exercise in rats. Braz J Med Biol Res. 2010;43(9):906–909. doi: 10.1590/s0100-879x2010007500086. [DOI] [PubMed] [Google Scholar]
- 19.de Cássia Cypriano Ervati Pinter R, Padilha AS, de Oliveira EM, Vassallo DV, de Fúcio Lizardo JH. Cardiovascular adaptive responses in rats submitted to moderate resistance training. Eur J Appl Physiol. 2008;103(5):605–613. doi: 10.1007/s00421-008-0761-3. [DOI] [PubMed] [Google Scholar]
- 20.Barauna VG, Junior ML, Costa Rosa LF, Casarini DE, Krieger JE, De Oliveira EM. Cardiovascular adaptations in rats submitted to a resistance-training model. Clin Exp Pharmacol Physiol. 2005;32(4):249–254. doi: 10.1111/j.1440-1681.2005.04180.x. [DOI] [PubMed] [Google Scholar]
- 21.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 22.Howley ET. Type of activity: Resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33(6):364–369. doi: 10.1097/00005768-200106001-00005. [DOI] [PubMed] [Google Scholar]
- 23.Børsheim E, Bahr R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003;33(14):1037–1060. doi: 10.2165/00007256-200333140-00002. [DOI] [PubMed] [Google Scholar]
- 24.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 25.Dunstan DW, Daly RM, Owen N, Jolley D, de Courten M, Shaw J, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 26.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or bothon glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–S30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 28.Lenzen S, Panten U. Alloxan: history and mechanism of action. Diabetologia. 1988;31(6):337–342. doi: 10.1007/BF02341500. [DOI] [PubMed] [Google Scholar]
- 29.Szkudelski T. The mechanism of alloxan and streptozotocin action in ß cells of the rat pancreas. Physiol Res. 2001;50(6):536–546. [PubMed] [Google Scholar]
- 30.Winterbourn CC, Munday R. Glutathione-mediated redox cycling of alloxan. Mechanisms of superoxide dismutase inhibition and of metal-catalyzed OH. formation. Biochem Pharmacol. 1989;38(2):271–277. doi: 10.1016/0006-2952(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 31.Jensen TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. J Physiol. 2012;590(5):1069–1076. doi: 10.1113/jphysiol.2011.224972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ropelle ER, Pauli JR, Carvalheira JBC. Efeitos moleculares do exercício físico sobre as vias de sinalização insulínica. Motriz. 2005;11(1):49–55. [Google Scholar]
- 33.Gross JL, Silveiro SP, Camargo LL. Diabetes mellito: diagnóstico, classificação do controle glicêmico. Arq Bras Endrocrinol Metab. 2002;46(1):16–26. [Google Scholar]
- 34.De Angelis K, da Pureza DY, Flores LJ, Rodrigues B, Melo KF, Schaan BD, et al. Efeitos fisiológicos do treinamento físico em pacientes portadores de diabetes tipo 1. Arq Bras Endocrinol Metab. 2006;50(6):1005–1013. doi: 10.1590/s0004-27302006000600005. [DOI] [PubMed] [Google Scholar]
- 35.Lash JM, Bohlen HG. Structural and functional origins of suppressed acetylcholine vasodilation in diabetic rat intestinal arterioles. Circ Res. 1991;69(5):1259–1268. doi: 10.1161/01.res.69.5.1259. [DOI] [PubMed] [Google Scholar]
- 36.Cosentino F, Luscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(Suppl 3):54–61. [PubMed] [Google Scholar]
- 37.Zguira MS, Vincent S, Le Douairon Lahaye S, Malarde L, Tabka Z, Saïag B. Intense exercise training is not effective to restore the endothelial NO-dependent relaxation in STZ-diabetic rat aorta. Cardiovasc Diabetol. 2013;12:32–32. doi: 10.1186/1475-2840-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faria T de O, Targueta GP, Angeli JK, Almeida EA, Stefanon I, Vassallo DV. Acute resistance exercise reduces blood pressure and vascular reactivity, and increases endothelium-dependent relaxation in spontaneously hypertensive rats. Eur J Appl Physiol. 2010;110(2):359–366. doi: 10.1007/s00421-010-1508-5. [DOI] [PubMed] [Google Scholar]
- 39.Chen SJ, Wu CC, Yen MH. Exercise training activates large-conductance calcium-activated K+ channels and enhances nitric oxide production in rat mesenteric artery and thoracic aorta. J Biomed Sci. 2001;8(3):248–255. doi: 10.1007/BF02256598. [DOI] [PubMed] [Google Scholar]
- 40.Halliwill JR. Mechanisms and clinical implications of postexercise hypotension in humans. Exerc Sports Sci Rev. 2001;29(2):65–70. doi: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]