Table 2.
Synthesis of Trisubstituted Oxazolidines.
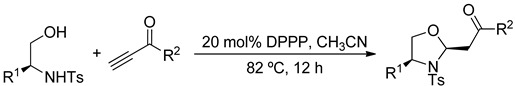
| Entry | R1 | R2 | Oxazolidine | Yield (%) |
|---|---|---|---|---|
| 1 | i-Pr | OMe | (2S, 4S)-3a | 59 |
| 2 | i-Pr | OEt | (2S, 4S)-3b | 75 |
| 3 | i-Pr | OBn | (2S, 4S)-3c | 63 |
| 4 | i-Pr | OPh | (2S, 4S)-3d | 22 |
| 5 | i-Pr | Me | (2S, 4S)-3e | 53 |
| 6 | i-Pr | Ph | (2S, 4S)-3f | 55 |
| 7 | i-Pr | m,p-Cl2C6H3 | (2S, 4S)-3g | 28 |
| 8 | i-Pr | m,p-(CH3O)2C6H3 | (2S, 4S)-3h | 58 |
| 9 | i-Pr | p-FC6H4 | (2S, 4S)-3i | 63 |
| 10 | i-Pr | 1-naphthyl | (2S, 4S)-3j | 70 |
| 11 | i-Pr | 2-thienyl | (2S, 4S)-3k | 37 |
| 12 | Me | OMe | (2S, 4S)-4a | 68 |
| 13 | Me | OEt | (2S, 4S)-4b | 68 |
| 14 | Me | OBn | (2S, 4S)-4c | 64 |
| 15 | Me | OPh | (2S, 4S)-4d | 14 |
| 16 | Me | Me | (2S, 4S)-4e | 68 |
| 17 | Me | Ph | (2S, 4S)-4f | 57 |
| 18 | Me | m,p-Cl2C6H3 | (2S, 4S)-4g | 41 |
| 19 | Me | m,p-(CH3O)2C6H3 | (2S, 4S)-4h | 90 |
| 20 | Me | p-FC6H4 | (2S, 4S)-4i | 27 |
| 21 | Me | 1-naphthyl | (2S, 4S)-4j | 33 |
| 22 | Me | 2-thienyl | (2S, 4S)-4k | 25 |
| 23 | Bn | OMe | (2S, 4S)-5a | 66 |
| 24 | Bn | OEt | (2S, 4S)-5b | 81 |
| 25 | Bn | OBn | (2S, 4S)-5c | 76 |
| 26 | Bn | OPh | (2S, 4S)-5d | 5 |
| 27 | Bn | Me | (2S, 4S)-5e | 45 |
| 28 | Bn | Ph | (2S, 4S)-5f | 71 |
| 29 | Bn | m,p-Cl2C6H3 | (2S, 4S)-5g | 30 |
| 30 | Bn | m,p-(CH3O)2C6H3 | (2S, 4S)-5h | 98 |
| 31 | Bn | p-FC6H4 | (2S, 4S)-5i | 51 |
| 32 | Bn | 1-naphthyl | (2S, 4S)-5j | 96 |
| 33 | Bn | 2-thienyl | (2S, 4S)-5k | 41 |
| 34 | CH2OH | OMe | (±)-6a | 38 |
| 35 | CH2OH | OEt | (±)-6b | 50 |
| 36 | CH2OH | OBn | (±)-6c | 37 |
| 37 | CH2OH | OPh | (±)-6d | 8 |
| 38 | CH2OH | Me | (±)-6e | 29 |
| 39 | CH2OH | Ph | (±)-6f | 23 |
| 40 | CH2OH | m,p-Cl2C6H3 | (±)-6g | 12 |
| 41 | CH2OH | m,p-(CH3O)2C6H3 | (±)-6h | 38 |
| 42 | CH2OH | p-FC6H4 | (±)-6i | 23 |
| 43 | CH2OH | 1-naphthyl | (±)-6j | 24 |
| 44 | CH2OH | 2-thienyl | (±)-6k | 58 |
