Abstract
VATER/VACTERL association refers to the non-random co-occurrence of the following component features: vertebral defects, anal atresia, cardiac malformations, tracheoesophageal atresia, renal abnormalities, and limb defects. Recently, Solomon et al. (Hum Genet 127:731–733, 2010) observed an increased prevalence of component features among first-degree relatives of VATER/VACTERL patients suggesting that in some patients, the disorder may be inherited. To replicate these findings, we investigated 87 VATER/VACTERL patients with the presence of a minimum of three component features and their first-degree relatives (n = 271). No increase in the overall prevalence of component features was observed in first-degree relatives compared to the general population (χ2 = 2.68, p = 0.10). Separate analysis for the prevalence of single component features showed a higher prevalence of tracheoesophageal fistula/atresia among first-degree relatives compared to the general population (OR 17.65, 95 % CI 2.47–126.05). However, this was based on occurrence in one family only. Our findings suggest that although familial occurrence renders a genetic contribution likely, the overall risk of recurrence among the first-degree relatives of patients with VATER/VACTERL association is probably very low. Since the patients in the present study were young and no offspring could be studied, estimation of the role of de novo mutations in the development of VATER/VACTERL was not possible.
Keywords: Inheritance, VATER, VACTERL, Association, First-degree relatives
Introduction
The acronym VATER was first introduced by Quan and Smith in 1973 to describe the non-random association of vertebral defects (V), anal atresia (A), tracheoesophageal fistula with or without esophageal atresia (TE), and radial dysplasia (R) [1]. The authors expanded the term 1 year later to include renal anomalies (R). In the same year, Temtamy et al. added cardiac (C) and limb defects (L), and the acronym was revised to VACTERL association [2, 3] (MIM #192350). Population-based epidemiological studies have reported an incidence of 1 in 10,000–1 in 40,000 live births [4, 5]. Recently, Solomon et al. [6] reported a significant increase in the prevalence of component features (CFs) among first-degree relatives of 78 patients with VATER/VACTERL association, which supports the hypothesis of the existence of a genetic background in some cases. To replicate this observation in an independent cohort, we investigated 87 patients, each of whom presented with at least three component features of the VATER/VACTERL association, and their first-degree relatives.
Methods
Patients
Between August 2009 and August 2011, European families with all forms of isolated and non-isolated anorectal malformations (ARM)/anal atresia were recruited by our consortium centers. Families were contacted through the German self-help organization for patients with ARM (SoMA e.V.) [7] and various pediatric surgery departments throughout Germany. Recruitment was carried out in person by one of four physicians, who were employed by the German Network for Congenital Uro-REctal Malformations (CURE-Net) and specifically trained in the diagnosis of the VATER/VACTERL association. For each proband, all available medical records were reviewed and their data were documented in a standardized case report form. When necessary, further clinical data were obtained from the responsible physician. Furthermore, a detailed family history was obtained. The study was approved by the respective local ethics committees, and informed consent was obtained from all participants.
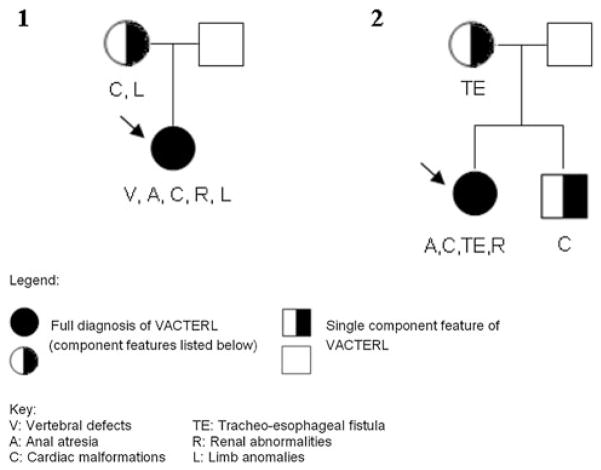
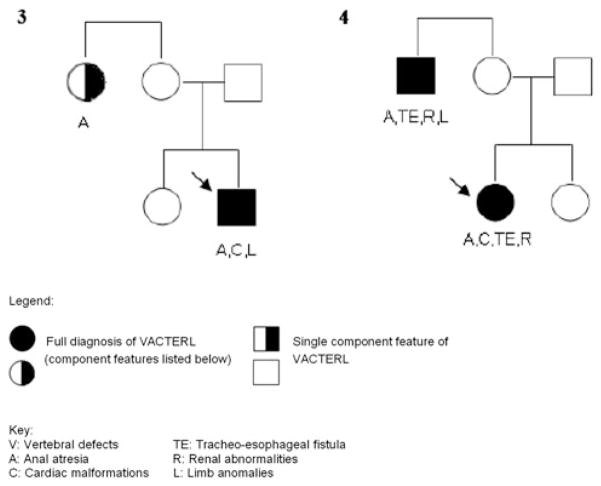
A total of 491 unrelated patients with ARM, born between 1954 and 2011, were recruited. Of these, 92 patients presented with at least two further CFs of the VATER/VACTERL association. Hence, all 92 patients were diagnosed with VATER/VACTERL association according to the commonly used conservative diagnostic criterion of the presence of at least three major CFs [1–3]. Congenital anomalies of the lower urinary tract, hypospadias, persistent ductus arteriosus, anomalies of the great arteries or veins, sacral dysplasia/agenesis, and neural tube defects were not considered to be CFs of the VATER/VACTERL association. Similarly, sacral dysplasia/agenesis was not considered to represent a vertebral defect CF, since all forms of ARM are associated with defects of the lower spine [8]. Furthermore, only limb malformations affecting the radial ray were considered to represent limb malformations in accordance with the clinical diagnostic criteria of the VATER/VACTERL association [1–3]. Patients were excluded if diagnoses other than VATER/VACTERL association were considered likely by the recruiting physician. Sufficient clinical information concerning all first-degree relatives was available for 87 of the 92 patients. These 87 patients had a total of 271 first-degree relatives (87 mothers, 87 fathers, 97 siblings). Of these 271 first-degree relatives, three presented with CFs of the VATER/VACTERL association spectrum (Families 1 and 2; Fig. 1; Table 1). In one family, a maternal aunt of the index patient presented with an ARM (Family 3; Fig. 2). In another family, the index patient and her maternal uncle presented with VATER/VACTERL association, both fulfilling the clinical criteria of the conservative definition (Family 4; Fig. 2). However, since the analysis was confined to first-degree relatives, the findings in the affected second-degree relatives in both these families (Family 3 and 4; Fig. 2) did not impact on the results of the present study.
Fig. 1.
Pedigrees of Family 1 and Family 2. Predigrees indicating major component features in the index patient (marked with an arrow) with VATER/VACTERL association and the first-degree relatives
Table 1.
Component features of VATER/VACTERL association in first-degree relatives
| Vertebral anomalies | Anal atresia/stenosis | Cardiac anomalies | Tracheoesophageal anomalies | Renal anomalies | Limb anomalies | |
|---|---|---|---|---|---|---|
| Mother (Family 1) | Pulmonic stenosis | Preaxial polydactyly | ||||
| Brother (Family 2) | Atrial septal defect type II | |||||
| Mother (Family 2) | Tracheoesophageal atresia | |||||
| Total | 0 | 0 | 2 (2.3 %) | 1 (1.1 %) | 0 | 1 (1.1 %) |
Fig. 2.
Pedigrees of Family 3 and Family 4. Pedigrees indicating major component features in the index patient (marked with an arrow) with VATER/VACTERL association and the affected second-degree relatives
Control cohort/general population
The control cohort comprised 1.4 million infants born between 1980 and 2009 who were assessed for the European Surveillance of Congenital Anomalies (EURO-CAT) registry.
Statistics
Fisher- and χ2-tests were used to compare the prevalence of CFs between first-degree relatives and the general population (EUROCAT registry [9]). Separate crude odds ratios (ORs) with 95 % confidence intervals (CIs) were calculated for each VATER/VACTERL CF. Since vertebral defects are not considered major birth defects by the International Clearinghouse for Birth Defects Surveillance and Research (ICDBSR) [10], no prevalence data for vertebral defects are available in the EUROCAT registry for comparison. In calculating the overall prevalence of the remaining CFs (A, C, TE, R, L), we conservatively assumed that these anomalies were independent of each other. All analyses were performed with the statistics software SAS©, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Analysis of first-degree relatives of patients with at least three CFs
No difference in the overall prevalence of CFs of the VATER/VACTERL association was observed between first-degree relatives and the general population (χ2 = 2.68, p = 0.10). A separate analysis for the prevalence of single CFs showed a significantly higher prevalence of tracheoesophageal fistula/atresia among first-degree relatives compared to the general population (OR 17.65, 95 % CI 2.47–126.05; Table 2). However, this was based on occurrence in one family only.
Table 2.
Comparison of the prevalence of component features of the VATER/VACTERL association between first-degree relatives of affected patients and the general population (EUROCAT 1980–2009)
| Odds ratio | 95 % confidence interval | p value | |
|---|---|---|---|
| Vertebral anomalies | – | – | – |
| Anal atresia/stenosis | – | – | – |
| Cardiac anomalies | 1.31 | 0.33–5.26 | 0.70 |
| Tracheoesophageal anomalies | 17.65 | 2.47–126.05 | 0.0042‡ |
| Renal anomalies | – | – | – |
| Limb anomalies | 4.58 | 0.64–32.58 | 0.13 |
The control cohort comprised 1.4 million infants born between 1980 and 2009 who were assessed for the European Surveillance of Congenital Anomalies (EUROCAT) registry
Statistically significant results are given in bold
Discussion
In 2010, Solomon et al. [6] described a significant increase in the prevalence of CFs among first-degree relatives of patients with VATER/VACTERL association. While Solomon et al. [6] used the relaxed inclusion criterion of a minimum of two CFs in their index patients, the present study required the presence of at least three CFs for the assignment of the diagnosis of VATER/VACTERL association, which is the more widely applied diagnostic criterion. Despite the use of this more strict inclusion criterion, the present study did not replicate the previous finding. Only tracheoesophageal fistula/atresia feature was more common among first-degree relatives of patients compared to the general population, and this finding was based on recurrence in a single family only.
Differences in the results of Solomon et al. [6] and the present study may also have been caused by differences in the control cohorts. Solomon et al. [6] compared a North-American patient cohort with approximately 3 million infants from the ECLAMC registry (Latin-American Collaborative Study of Congenital Malformations) [11]. The latter was ascertained between 1967 and 1990 in Latin-American countries, and no ultrasound screening for congenital renal or cardiac defects was performed during the early stages of the ascertainment of the ECLAMC cohort. Hence, the true prevalence of renal or cardiac defects in this cohort may be considerably higher than that determined at the time of ascertainment. The present study used a more recent control cohort, in which ultrasound screening for congenital renal or cardiac defects had been performed. However, vertebral defects are not considered major birth defects by the ICDBSR, and hence no prevalence data for vertebral defects were available. Similarly, no information concerning possible vertebral pathology was available for the majority of the first-degree relatives, and thus the VATER/VACTERL association spectrum CF “vertebral defects” was excluded from the present analyses.
In conclusion, our data suggest that the overall risk of recurrence among first-degree relatives of patients with VATER/VACTERL association is probably very low. Nevertheless, familial occurrence is observed in some rare families, as illustrated by various case reports. In the present sample, we observed one family in which a second-degree relative also fulfilled the conservative diagnostic criterion for VATER/VACTERL. We reported this family in a previous study and provided a systematic review of case reports of familial cases (Hilger et al. in press). A limitation of the present study in terms of the estimation of genetic influences on the development of VATER/VACTERL is the lack of offspring of our young patient cohort. Therefore, it remains possible that in a substantial proportion of VATER/VACTERL patients, the disease is caused by a de novo mutation. Investigation of this hypothesis will require other approaches, such as array-based searches for copy number variation and next-generation sequencing to detect smaller changes in the nucleotide sequence.
Acknowledgments
E.B., E.J., M.L., E.S., S.G.-D., S.M., S.H.-C., M.M.N., H.R., and N.Z. are members of the “Network for the Systematic Investigation of the Molecular Causes, Clinical Implications, and Psychosocial Outcome of Congenital Uro-Rectal Malformations (CURE-Net)”, which is supported by a research grant (01GM08107) from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF). We thank all the family members for their cooperation, and the German self-help organization for people with anorectal malformations (SoMA e.V.) for their assistance with recruitment. We thank Dr. Christine Schmael for her expert advice on the manuscript.
Footnotes
Conflict of interest The authors have no conflicts of interest to declare.
Contributor Information
Enrika Bartels, Email: enrika.bartels@uni-bonn.de, Institute of Human Genetics, University of Bonn, Bonn, Germany.
Ekkehart Jenetzky, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany.
Benjamin D. Solomon, Medical Genetics Branch, National Human Genome Research Institute, Bethesda, MD, USA
Michael Ludwig, Department of Clinical Chemistry and Clinical Pharmacology, University of Bonn, Bonn, Germany.
Eberhard Schmiedeke, Institute of Human Genetics, University of Bonn, Bonn, Germany. Department of Pediatric Surgery and Urology, Center for Child and Adolescent Health, Hospital Bremen-Mitte, Bremen, Germany.
Sabine Grasshoff-Derr, Department of Pediatric Surgery, University Hospital Würzburg, Würzburg, Germany.
Dominik Schmidt, Department of Pediatric Surgery, Campus Virchow Clinic, Charite University Hospital Berlin, Berlin, Germany.
Stefanie Märzheuser, Department of Pediatric Surgery, Campus Virchow Clinic, Charite University Hospital Berlin, Berlin, Germany.
Stuart Hosie, Department of Pediatric Surgery, Klinikum Schwabing, Technische Universität München, Munich, Germany.
Sandra Weih, Department of Pediatric Surgery, University of Heidelberg, Heidelberg, Germany.
Stefan Holland-Cunz, Department of Pediatric Surgery, University of Heidelberg, Heidelberg, Germany.
Markus Palta, Department of Pediatric Surgery, Evangelisches Krankenhaus Hamm, Hamm, Germany.
Johannes Leonhardt, Department of Pediatric Surgery, St. Bernward-Hospital, Hildesheim, Germany.
Mattias Schäfer, Department of Pediatric Surgery and Urology, Cnopf́sche Klinik, Nürnberg, Germany.
Christina Kujath, Department of Pediatric Surgery, University of Greifswald, Greifswald, Germany.
Anke Riβmann, Malformation Monitoring Centre Saxony-Anhalt, Otto-von-Guericke University, Magdeburg, Germany.
Markus M. Nöthen, Institute of Human Genetics, University of Bonn, Bonn, Germany. Department of Genomics, Life and Brain Center, University of Bonn, Bonn, Germany
Heiko Reutter, Institute of Human Genetics, University of Bonn, Bonn, Germany. Department of Neonatology, Children’s Hospital, University of Bonn, Bonn, Germany.
Nadine Zwink, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany.
References
- 1.Quan L, Smith DW. The VATER association. Vertebral defects, anal atresia, T-E fistula with esophageal atresia, radial and renal dysplasia: a spectrum of associated defects. J Pediatr. 1973;82:104–107. doi: 10.1016/s0022-3476(73)80024-1. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman RL. Birth defects and oral contraceptives. Lancet. 1973;1:1396. doi: 10.1016/s0140-6736(73)91731-5. [DOI] [PubMed] [Google Scholar]
- 3.Temtamy SA, Miller JD. Extending the scope of the VATER association: definition of the VATER syndrome. J Pediatr. 1974;85:345–349. doi: 10.1016/s0022-3476(74)80113-7. [DOI] [PubMed] [Google Scholar]
- 4.Botto LD, Khoury MJ, Mastroiacovo P, Castilla EE, Moore CA, Skjaerven T, et al. The spectrum of congenital anomalies of the VATER association: an international study. Am J Med Genet. 1997;71:8–15. doi: 10.1002/(sici)1096-8628(19970711)71:1<8::aid-ajmg2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Khoury MJ, Cordero JF, Greenberg F, James LM, Erickson JD. A population study of the VACTERL association: evidence for its etiologic heterogeneity. Pediatrics. 1983;71:815–820. [PubMed] [Google Scholar]
- 6.Solomon BD, Pineda-Alvarez DE, Raam MS, Cummings DA. Evidence for inheritance in patients with VACTERL association. Hum Genet. 2010;127:731–733. doi: 10.1007/s00439-010-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SoMA e. V. German self-help organisation for people with anorectal malformations (Internet) Munich, Germany: 2012. Available from: http://www.soma-ev.de. [Google Scholar]
- 8.Levitt MA, Peña A. Anorectal malformations. Orphanet J Rare Dis. 2007;2:33. doi: 10.1186/1750-1172-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EUROCAT. European surveillance of congenital anomalies (Internet) Co Antrim; Northern Ireland: 2012. Available from: http://www.eurocat-network.eu. [Google Scholar]
- 10.ICBDSR. International clearinghouse birth defects surveillance and research (Internet) Rome, Italy: 2012. Available from: http://www.icbdsr.org. [Google Scholar]
- 11.Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63:529–536. doi: 10.1002/(SICI)1096-8628(19960628)63:4<529::AID-AJMG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]