Figure 6. dSdhaf3 function is required in the muscles and nervous system.
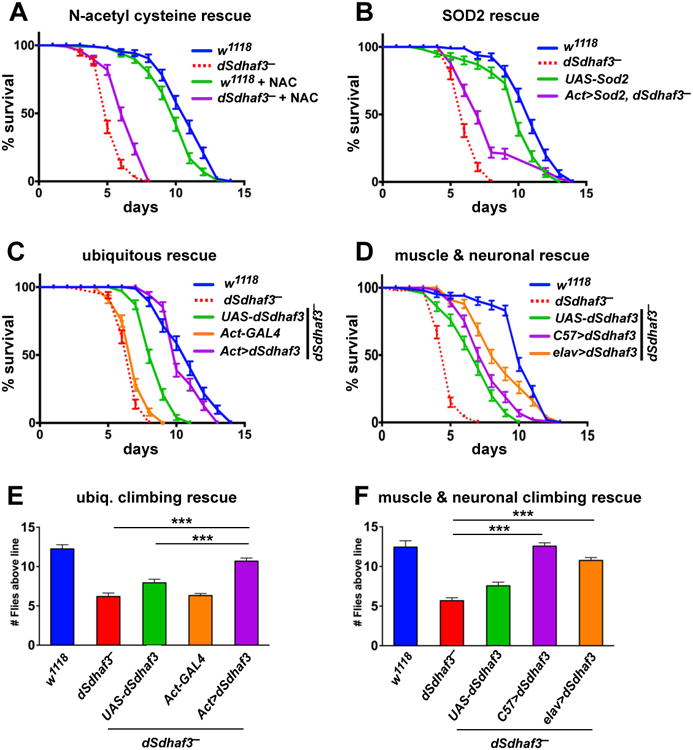
Five-day old w1118 control (blue solid line) and dSdhaf3 mutant (red dotted line) males were transferred to vials with 100% O2 with standard medium, and living animals were scored daily. (A) 0.1% N-acetyl cysteine was added to the culture medium for a quarter of the vials. (B) Expression of Sod2 using the ubiquitous Act5C-GAL4 driver (Act>Sod2; purple line) partially rescues the hyperoxia sensitivity of dSdhaf3 mutants. Both NAC treatment and Sod2 expression significantly rescue the hyperoxia sensitivity of dSdhaf3 mutants, p<0.001. (C) Expression of wild-type dSdhaf3 using the ubiquitous Act5C-GAL4 driver (Act>dSdhaf3; purple line) rescues the hyperoxia sensitivity of dSdhaf3 mutants (p<0.001). (D) The muscle-specific C57-GAL4 driver provides minor, but significant (p<0.01), rescue of the hyperoxia sensitivity of dSdhaf3 mutants (purple line) relative to the control that carries the UAS-dSdhaf3 transgene alone (green line), while the CNS-specific elav-GAL4 driver provides more efficient rescue (orange line)(p<0.001). Expression of wild-type dSdhaf3 by using either the ubiquitous Act5C-GAL4 driver (E, purple), the muscle-specific C57-GAL4 driver (F, purple), or the CNS-specific elavGAL4 driver (F, orange) rescues the climbing defect in dSdhaf3 mutants. The Act>dSdhaf3 rescue in C and E was performed in females, other rescue studies were performed in males (A,B,D,F). The apparent partial rescue of dSdhaf3 mutants by a single copy of the UAS-dSdhaf3 transgene (C,D, green line) appears to be due to genetic background since UAS-dSdhaf3 transformants have normal levels of SdhB and SDH activity (E,F). Each graph was compiled from two experiments with a total of 10 vials with 20 animals per vial. ***p<0.001
