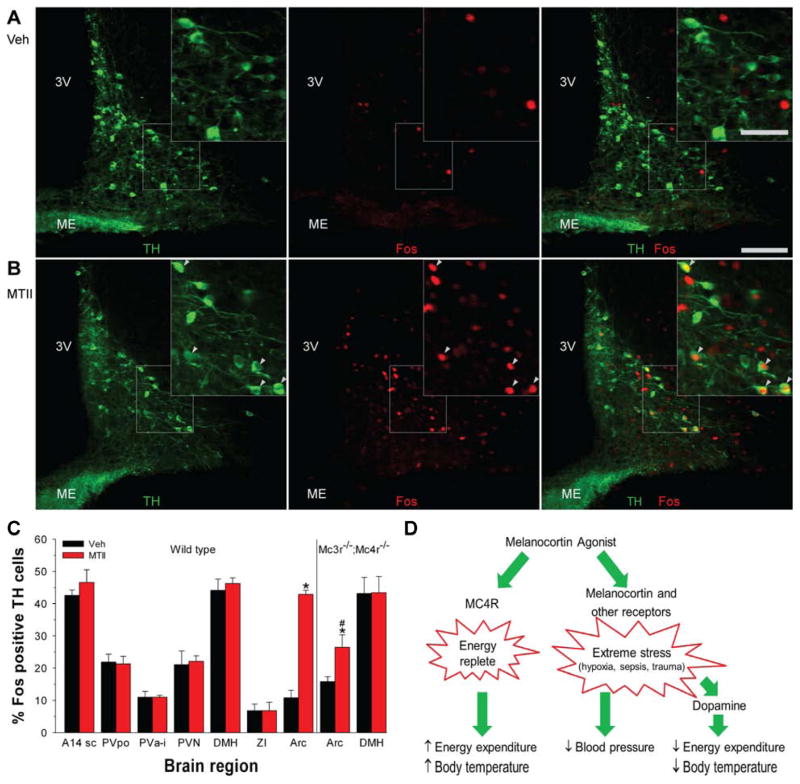
Figure 7.
MTII activates dopaminergic neurons selectively in the arcuate nucleus. A, B) Immunohistochemistry for TH (green; left panel) and Fos (red; middle panel) after vehicle (A) or MTII (B) treatment of C57BL/6J mice. Scale bar is 100 μm in main image and 50 μm in insert. Arrowheads indicate Fos-positive TH neurons; 3V, third ventricle; ME, median eminence. (C) Percentage TH-immunoreactive neurons staining for Fos after vehicle or MTII administration in wild type (n = 3/group) and Mc3r−/−;Mc4r−/− mice (n = 4/group). Neurons were counted in the subcommissural part of A14 dopaminergic cell group (A14sc; 2–3 sections), preoptic part of the periventricular hypothalamic nucleus (PVpo; 3 sections), paraventricular nucleus of the hypothalamus (PVN; 6–7 sections), anterior and intermediate part of the periventricular hypothalamic nucleus (PVa-i; 6–7 sections), dorsomedial hypothalamic nucleus (DMH; 2–3 sections), zona incerta (ZI; 3 sections), and arcuate nucleus (Arc; 6–7 sections). The number of TH neurons per region were not statistically different between the MTII and vehicle groups and the analyzed areas had expected distributions of TH-immunoreactive neurons (Lookingland and Moore, 2005). Data are mean ±SEM; * indicates P<0.05 vs vehicle. # indicates P<0.05, wild type vs Mc3r−/−;Mc4r−/−. (D) Actions of melanocortin agonists. Melanocortin agonists act via Mc4r to signal that the body is in an ‘energy replete’ state, detectable for example as an increase in energy expenditure and Tb during fasting. Melanocortin agonists also act likely via both melanocortin and other receptors to signal ‘extreme stress’ such as caused by hypoxia, sepsis, and severe trauma. This triggers a time-limited set of responses including hypometabolism and hypothermia mediated by dopaminergic pathways and also hypotension, which appears not to occur via dopaminergic pathways.