Fig. 1.
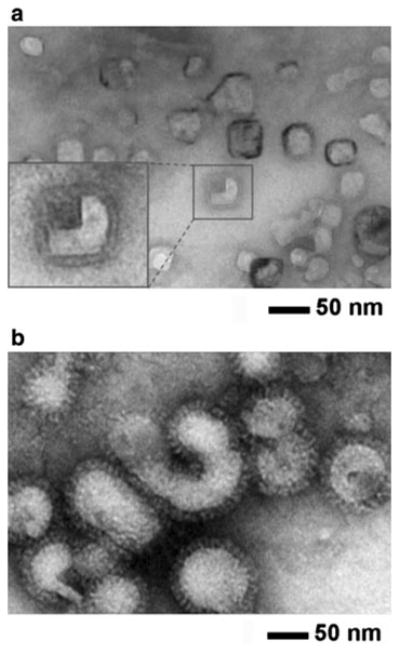
Negative-stain TEM micrographs of influenza vaccine particles. Inactivated A/PR/8/34 (H1N1) influenza virus vaccines. a split vaccine particles and b whole inactivated virus (WIV) vaccine particles. Vaccines incubated in iso-osmotic sucrose medium (pH 7.0, 4 °C) were stained with phosphotungstic acid (2 %, pH 7.0) and allowed to dry under ambient conditions before imaging. Inset at the left bottom in a shows magnified split vaccine image. Vaccine particle size was determined by TEM image analysis for split vaccine particles (30 ± 6 nm in diameter, n = 30) and WIV vaccine particles (87 ± 17 nm in diameter, n = 80)
