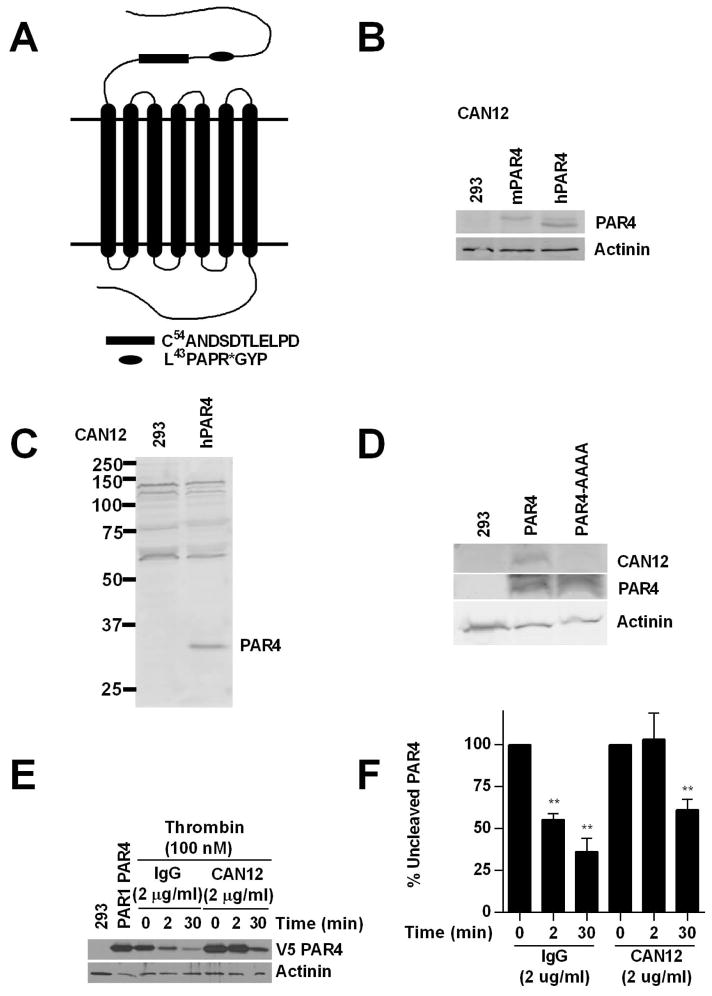
Figure 1. CAN12 reduces the rate of PAR4 cleavage.
(A) The N-terminus of PAR4 with C54ANDSDTLELPD (CAN12) and the thrombin cleavage site (*) indicated. (B) HEK293 cells (293) or cells transfected with mouse PAR4 (1.0 μg) or human PAR4 (1.0 μg) blotted with CAN12 (1:100) and actinin (1:1000). (C) Full gel for CAN12 interacting with hPAR4 expressed in HEK293 cells. (D) HEK293 cells (293) or cells transfected with hPAR4 (4.0 μg) or hPAR4-AAAA (D57A, D59A, E62A, D65A) (4.0 μg) blotted with CAN12, PAR4 (C-10), and actinin. (E) HEK293 cells transfected with HA-hPAR1 (4.0 μg) and V5-hPAR4 (0.1 μg) pretreated with buffer (PAR1PAR4), IgG (2 μg/ml), or CAN12 (2 μg/ml) for 10 min at room temperature. The cells were then activated with thrombin (100nM) for 0, 2, or 30 min at 37°C. Cleavage of PAR4 was measured by loss of the N-terminal V5 epitope. (F) Quantitation of percent of uncleaved PAR4 compared to 0 min as 100%. n=4 **p<0.01 vs. time zero