Abstract
Many RNAs present unique challenges in obtaining material suitable for structural or biophysical characterization. These issues include synthesis of chemically and conformationally homogeneous RNAs, refolding RNA purified using denaturing preparation techniques, and avoiding chemical damage. To address these challenges, new methodologies in RNA expression and purification have been developed seeking to emulate those commonly used for protein purifications. In this review, recent developments in the preparation of high-quality RNA for structural biology and biophysical applications are discussed, with an emphasis on native methods.
Introduction
To date, RNA structural biology has been largely dominated by small, highly organized elements such as ribozymes, aptamers and isolated sequence motifs [1]. Generally, these molecules pose few challenges to obtaining the milligram quantities of chemically and conformationally homogeneous material required for structural analysis. Almost all of these RNAs were prepared using the classic workflow of synthesis by in vitro transcription using T7 RNA polymerase (RNAP) and purification by denaturing polyacrylamide gel electrophoresis (PAGE) followed by refolding (this methodology is described in detail in [2]). However, for many RNAs, this approach does not yield RNAs in biologically relevant conformations, have key post-transcriptional modifications or assemble into a functional ribonucleoprotein (RNP) particle.
The standard method for RNA preparation can fail for a number of reasons. As the size of the desired RNA increases, it becomes progressively more difficult to separate transcription products that differ by a few nucleotides. This problem is particularly acute for T7 RNAP transcription, which is known to introduce chemical heterogeneity at both the 5′- and 3′-ends [3,4]. Another important consideration is that, while simple, denaturing PAGE purification is usually a two or three day protocol with limited throughput. For applications requiring surveying a large number of RNAs, this introduces a significant bottleneck [2,5]. Further, it is not always the case that the desired RNA can be refolded. Even seemingly simple RNAs have been observed to misfold with popular “heat/cooling” techniques [6]. The separation process, particularly visualizing of RNA in a polyacrylamide gel by UV shadowing, can damage RNA in ways making it unsuitable for applications like single molecule spectroscopy [7,8]. Further, the polyacrylamide gel can introduce contaminants that are difficult to remove and interfere with NMR spectroscopy [9]. To meet these challenges, a new generation of techniques is emerging addressing a number of pitfalls of the classic RNA purification methodology with a particular emphasis on native expression and purification. In this review, I present a survey of these new methods and their utility for resolving specific issues.
In vivo expression of RNA using the “tRNA scaffold”
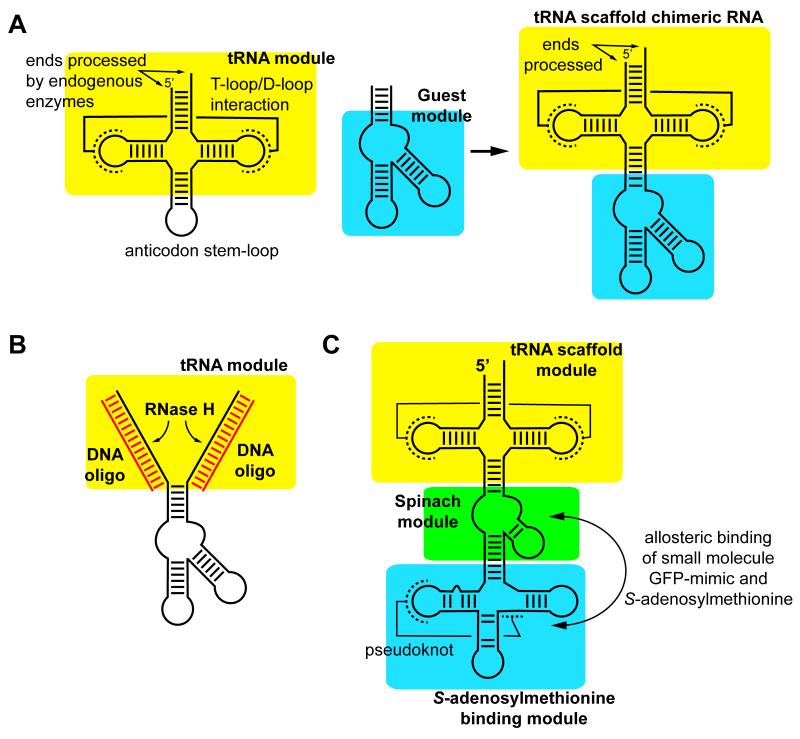
While in vitro transcription T7 RNAP [10] remains the preferred method for synthesis of a defined RNA sequence, an alternative approach has been developed enabling expression in E. coli. Production of RNA in vivo is generally considered problematic because of issues relating to transcript heterogeneity, degradation from intracellular RNases and difficulty purifying the product from cellular extracts [11]. Dardel and Ponchon provide an elegant solution to these problems with the “tRNA scaffold” [11]. Endogenous enzymes efficiently process the 5′- and 3′-ends of tRNA to yield a uniform product with a highly stable structure resistant to unfolding and degradation. To exploit these properties, the desired RNA sequence is inserted into the anticodon stem-loop creating a chimeric RNA (Figure 1A). The host tRNA component of the chimera still adopts the core three-dimensional structure of tRNA, while the guest retains its distinct structural and functional properties. Several expression vectors have been developed enabling facile cloning of a desired sequence into the context of E. coli tRNAMet or human tRNALys3 placed downstream of a constitutive lpp promoter [11]. This method has been used to express a series of RNAs from small hairpins (~30 nucleotides) to the 5′-domain of the E. coli 23S rRNA (376 nucleotides) [11,12]. Product RNA is obtained by phenol extraction and passaging the aqueous phase over an anion exchange column followed by gel filtration [12]. In a useful variation of this method, the tRNA scaffold expression plasmid can be co-transfected with a compatible protein expression vector to yield an RNP particle [13].
Figure 1.
The tRNA scaffold for in vivo expression of RNA. (A) The structured core of tRNA (yellow) is used as a host for an RNA of interest (cyan) which are fused together through replacement of the tRNA anticodon stem-loop to create the chimera. (B) Removal of the scaffold from the RNA of interest can be achieved by DNA oligonucleotides hybridizing to both sides of the tRNA and cleaving the duplex with RNase H. (C) More complex chimeras can be created that incorporate multiple modules to create an in vivo sensor of small molecule metabolites such as S-adenosylmethionine.
A downside of the above method is the product RNA retains the host tRNA element, which may not be desirable for biochemical or structural analysis. To excise the guest RNA, an approach has been developed that anneals DNA oligonucleotides to the host tRNA portions of the chimera and the RNA portion of the DNA/RNA hybrid degraded by RNase H (Figure 1B) [14]. However, any native fold of the guest RNA is destroyed by the heat/cooling protocol for hybridizing the DNA oligonucleotides to the tRNA scaffold, which can be a problem if fully native purification is desired. To circumvent this problem, an alternative is to use hammerhead ribozymes placed at the 5′- and 3′-sides of the RNA sequence of interest for processing [15]. While this eliminates the need for enzymatic RNase H digestion, it requires a further denaturing polyacrylamide gel purification step to fully purify the product.
Modified RNAs are particularly difficult to access in the quantities necessary for structural characterization. Generally, this requires chemical synthesis of the desired RNA, and in particular synthesis of the modified nucleotide(s) as appropriately protected and activated precusors [16,17]. However, the tRNA scaffold enables expression of modified RNAs. In a variation of the RNP expression protocol, human tRNALys3 was coexpressed with T. thermophilus TrmI, an enzyme that produces the m1A58 modfication. Analysis of product RNA by NMR revealed the appropriate adenosine was efficiently methylated at the N1 position when coexpressed with TrmI, opening the door for simple and efficient expression of modified RNAs [13].
A potentially important application of the tRNA scaffold methodology is stabilizing in vivo RNA biosensors. The Jaffrey group developed an RNA aptamer called “Spinach” that specifically binds a cell-permeable molecule mimicking the fluorophore of GFP that becomes fluorescent upon binding the aptamer [18]. This aptamer was used as part of a chimeric RNA comprising the tRNA scaffold, Spinach aptamer and an aptamer domain from an S-adenosylmethionine (SAM) binding riboswitch (Figure 1C). When expressed in E. coli, this chimera enabled the real-time monitoring of intracellular SAM levels [18]. Similar biosensors for cyclic-di-GMP [19,20] and proteins [21] have also been developed indicating a potentially broad utility of this approach for cellular imaging. These and other potential applications make it highly likely the tRNA scaffold methodology will have a significant impact on the RNA field beyond expression.
Affinity tag purification methods
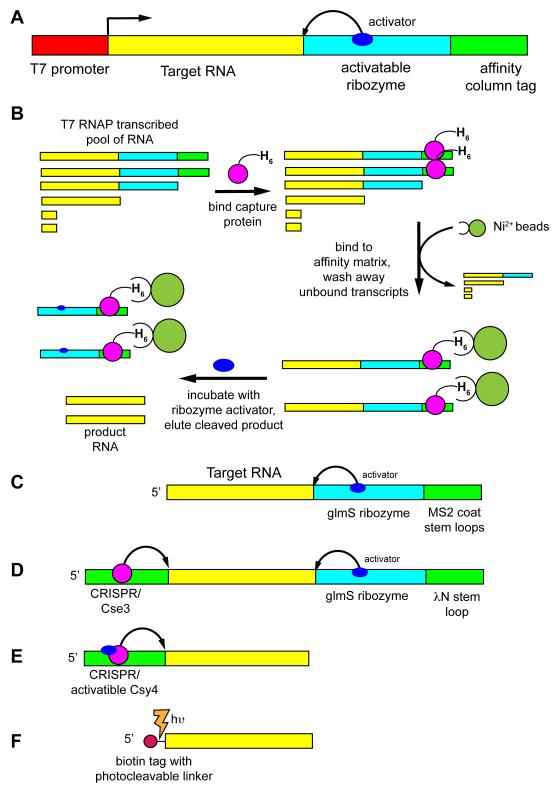
Over the past decade a number of native purification techniques have emerged, motivated by problems associated with denaturing polyacrylamide gel electrophoresis (PAGE). The first significant effort to generate a universal native RNA purification method sought to emulate protein affinity tags that revolutionized routine and rapid protein purification [22]. This approach generally requires two components to be added to the RNA of interest: a capture module and an activatable ribozyme (Figure 2A). Since transcription by T7 RNAP yields a substantial amount of short abortive products, an affinity capture tag must be placed at the extreme 3′-end of the transcript to partition these smaller RNAs away from full-length product. Together, these modules enable the general purification scheme illustrated in Figure 2B. Like protein purification, a number of affinity tags have been developed enabling users to choose the type most suited to their needs or easiest to implement.
Figure 2.
Schematic of various native affinity tag purification methods. (A) The general scheme involves creating a dsDNA template for use in a T7 RNAP transcription reaction that contains a promoter element (red), the RNA of interest on the 5′-end of the transcript (yellow), an activatable ribozyme that cleaves at the 3′-end of the RNA of interest (cyan) and an affinity tag at the extreme 3′-end of the transcript (green) for selective immobilization of the full-length transcript to a column. (B) Purification of the RNA of interest in the variation using a hexahistidine-tagged MS2 coat binding protein comprises adding the capture protein to the transcription reaction and allowing it to bind, passing the reaction over a nickel affinity column to bind full-length transcript, washing away other transcription reaction components, followed by eluting the RNA of interest by incubating the column with an effector that activates the ribozyme [22,37]. Variations of this methodology use different combination of cleavage methods and tags, including (C) glmS/MS2 coat [37], (D) the “ARiBo” tag using both a 3′- glmS/λN peptide module and a 5′ Cse3 tag [38], (E) an activatable Cys4-binding module at the 5′-end that is used for both immobilization and cleavage [36], and (F) a 5′ photocleavable biotin tag [46].
First, the RNA can be tagged with a specific sequence that hybridizes to a DNA oligonucleotide. This is the simplest means of capturing a nucleic acid and is commonly used for the purification of poly-adenylated mRNAs with oligo(dT) beads [23]. Walter and coworkers have developed a variant of this method in which the 3′-end of the RNA transcript hybridizes to a DNA oligonucleotide biotinylated at its 5′-end [24]. The RNA/DNA hybrid is captured using magnetic streptavidin beads, allowing for other transcription components to be washed away. Notably, in this study it was demonstrated RNAs purified using native methods avoid the conformational heterogeneity problems and loss of activity associated with refolding by heat/cooling methods [24].
The second type of capture tag used is a module recognizing a small molecule or polymer, such as the streptavidin [25] and Sephadex [26] aptamers, respectively. Each of these sequences specifically bind a commercially available resin, making them an easy route for capturing RNAs. These tags have clear advantages and limitations [27]. Weak binding and the requirement of high molecular weight dextran for competitive elution strongly reduce the practicality of the Sephadex aptamer. Conversely, the high affinity for streptavidin (70 nM) and the ability to elute with biotin makes the streptavidin aptamer a useful affinity capture tag that has been increasingly employed for purification of RNP particles from cells [28-30].
The most well-developed affinity tags currently in use employ RNA-binding proteins. In this embodiment the RNA component bears a small protein recognition element, while its partner has a tag such as hexahistidine for capture by an affinity matrix. The primary rationale for using proteins is that they exhibit high affinity and specificity for their target, and perhaps more importantly, generally have lower dissociation rates as compared to small molecule binding aptamers. The most broadly used tagging protein for RNA is the MS2 coat protein that specifically binds a small hairpin motif [31]. This protein-RNA interaction has been applied to a number of in vitro and in vivo applications and has proven robust [32-34]. Other protein binding tags implemented in native affinity purification schemes include the bacterial signal recognition particle [22], a short peptide derived from the lambda phage N-protein [35], and a conditionally active Csy4 endonuclease [36].
In addition to capturing the tagged RNA, eluting RNA from the column-bound tag constitutes the second necessary requirement for affinity tag purification. Generally, this is accomplished with a ribozyme cleaving at its 5′-end to liberate product RNA from the ribozyme/affinity tag component (Figure 2A). Two ribozymes have been employed in various affinity purification methods: an imidazole-activated mutant hepatitis delta virus (HδV) ribozyme [22] and the glmS ribozyme activated by glucosamine-6-phosphate (Gln6P) [24,35,37,38]. Both ribozymes have minimal sequence requirements on the product RNA side of their cleavage site, making them compatible with almost any RNA. The primary drawbacks of the HδV ribozyme are the requirement for a high concentration of imidazole (~500 mM) to activate cleavage and slow rate of cleavage (~2 hours at 37 °C), which can lead to significant degradation of the product RNA. Conversely, the natural glmS ribozyme rapidly cleaves (~10 minutes) to completion in low concentrations of Gln6P (1 mM), but is incompatible with Tris buffer, which activates the ribozyme [39]. Besides ribozymes, a mutant form of the Csy4 protein can be used to cleave on the 3′-side of a small hairpin placed at the 5′-end of the transcript [36]. After immobilization of the biotinylated Cys4-RNA complex with streptavidin beads, addition of 500 mM imidazole activates cleavage by the enzyme. This liberates an RNA product with a uniform 5′-end from the column, but the product RNA still retains any 3′-end heterogeneity in the precursor. The other disadvantage of this system is again the use of high imidazole concentrations and slow cleavage rates (requires overnight incubation). Together, various capture and/or cleavage modules have been arranged to yield different affinity purification tags (Figure 2C-F).
5′-end chemical tagging
Another method for tagging RNA transcripts requires attachment of a small molecule to the 5′-end that is captured using an affinity resin. T7 RNA polymerase can initiate transcription with GMP along with di- and tri-nucleotides such as pppGpG that are a result of abortive initiation [4]. By extension, the initiating nucleotide can host a variety of groups attached to the 5′-hydroxyl and be exclusively incorporated into the 5′-position of the transcript [40-43]. To incorporate adenosine derivatives as the initiating nucleotide in T7 RNAP transcription, the alternative class II ϕ2.5 promoter must be used [44] as opposed to the class III ϕ6.5 promoter that requires guanosine [45].
Taking advantage of this observation, Dayie and Sintim developed a method to couple a caged photocleavable biotin moiety to GMP (referred to as biotin-PC GMP) using simple click chemistry [46]. To maximize incorporation of biotin-PC GMP, the overall NTP concentrations in the transcription reaction need to be optimized, as well as the ratio of modified GMP to GTP. For a model RNA derived from a group II intron, the authors found that they achieved a 17% incorporation of biotin-PC GMP at a 4:1 ratio of biotin-PC GMP:GTP at 5 mM total NTP concentration. 5′-end labeled RNA in the resultant transcription reaction is purified using an avidin column following removal of unincorporated GMP derivative. Product RNA is liberated from the column by irradiating the resin with UV light at 365 nm for one hour. To limit 3′-end heterogeneity of the transcript, the DNA template has 2′-O-methyl modifications at its 3′-end on the coding strand [47,48].
SEC and anion exchange column purification
Within the last few years several methods have emerged that exploit size exclusion chromatography (SEC) or weak anion exchange chromatography. These techniques have distinct advantages and disadvantages over the tagged purification methods described above. As a major advantage, these methods do not require a significant fraction of the transcript be dedicated to a cleaved tag, which impacts overall yield. This is a particularly important concern for structural applications requiring expensive 13C and/or 15N-labeled NTPs. Also, these methods are easily automated, making them less labor and time intensive, another significant concern for applications requiring testing many RNA sequences. One substantial downside is the resolution of these methods because they cannot resolve closely related species, such as the commonplace 3′-end heterogeneity, and may not be completely compatible with ribozyme-mediated 5′- and 3′-end processing used to achieve chemical homogeneity. However, used in tandem, affinity, SEC and anion exchange chromatography can achieve very high chemical and conformational purity of a desired RNA, just as for proteins.
Size exclusion chromatographic techniques were originally developed to circumvent PAGE purification resulting in polyacrylamide contaminants that complicate NMR analysis [9]. Improvements in the first inception of this method have made this a rapid and practical method to purify milligram quantities of RNA [49,50]. Importantly, the Superdex-75 and -200 columns have been calibrated with a set of standard RNAs of various lengths to determine the separation characteristics of each. While each RNA differs in shape as well as size, this information provides a reasonable starting point for choosing the appropriate column for the desired RNA. An important feature of this chromatographic method over other native methods is the ability to purify away multimeric and aggregated species and potentially even different conformers. This is a particularly common problem in RNA, which has a marked tendency to multimerize, such as short hairpins that are mostly self-complimentary.
The technique of native purification that is emerging as a favorite in our research efforts is weak anion exchange chromatography. Lukavsky and coworkers have developed a facile method for rapidly purifying RNA from transcription reactions by FPLC with a DEAE-Sepharose FastFlow column [51,52]. One of the primary advantages of this separation technique is the crude transcription reaction can be loaded directly onto to the column without any prior workup. Separation of unincorporated NTPs, enzyme and other transcription reaction components from the product RNA is achieved using a standardized NaCl gradient. Eluted product is subsequently buffer exchanged and concentrated in centrifugal microconcentrators. For most biophysical applications, the quality and homogeneity of RNA purified by this method is sufficient without further purification. This technique has the further advantages of generating quantities of RNA necessary for NMR spectroscopy or X-ray crystallography, requiring equipment and techniques available to any biochemistry laboratory, and is very rapid (2-4 hours) compared to other methods.
Case study of a very challenging RNA: the group II intron
An emerging challenge for structural studies are large RNAs (>500 nucleotides) that present new issues for synthesis and purification. First, it is well known that RNAs often have a rugged folding landscapes [53]. As the size of the RNA increases, traditional techniques for refolding the RNA such as heat/cooling are likely to become problematic, although the ~800 nucleotide SRA RNA was successfully refolded into a homogeneous secondary structure in this fashion [54]. Second, large RNAs are more likely to rely upon co-transcriptional folding to access the correct conformation. Even small model RNAs containing mutually exclusive and nearly isoenergetic secondary structures can exhibit folding pathways that are dictated by transcription [55]. Furthermore, RNPs such as the bacterial 30S ribosome assemble in vivo and in vitro assemble with a distinct 5′-to-3′ polarity, reflecting co-transcriptional RNA folding and RNP assembly (reviewed in [56]). Since there is a kinetic component to co-transcriptional folding, it may also be that T7 RNA polymerase may transcribe too rapidly to allow the transcript to fully explore this folding landscape. Wildtype T7 RNAP under in vitro transcription conditions synthesizes RNA at a rate of ~240 nucleotides (nt) per second, significantly faster than cellular RNAPs (~30-90 nt/sec [57] for bacterial and ~7 nt/sec for mammalian RNAP II [58]). In Escherichia coli, there is an inverse correlation between polymerase speed and amount of β-galactosidase produced per transcript, suggesting rapid transcription can lead to unproductive folding [59]. Slower polymerases such as mutant T7 RNA polymerases (40-160 nt/sec) might be desirable in these cases [60,61].
An example of the care that large RNAs require in their purification to preserve native architecture is highlighted by studies to determine the crystal structure of a group II self-splicing intron [62]. From extensive folding studies, it was established that model group II introns can be difficult to fold [63], making it difficult to obtain the conformationally pure samples required for crystallization. This issue was resolved in several ways. First, a large set of group II intron variants was screened to find one that efficiently self-spliced under low magnesium and high temperature conditions [62]. The best sequence, derived from an intron in Oceanobacillus iheyensis, was part of a class of group II introns smaller in size and more reactive such that it efficiently self-spliced during in vitro transcription. Screening “sequence space” for a highly functional or crystallizable RNA is a highly successful strategy in RNA structural biology [5]. Despite these advantages, this RNA could not be efficiently refolded once denatured, as indicated by native gel analysis [62]. To circumvent this issue, the template DNA and polymerase was destroyed with DNase and proteinase K, respectively, and the remaining RNA buffer exchanged in a 100 kDa molecular weight cut-off centrifugal concentrator to remove smaller impurities [62]. This treatment removed all components of the transcription, including the spliced exons, while retaining the spliced product intron that was then used for crystallization. Other tools used for the purification of large RNP complexes such ultracentrifugation may also be increasingly implemented to purify and analyze RNAs that is nondestructive to its native fold [64].
Conclusion
Despite the dramatic increase in the number of RNA expression and purification methodologies developed over the last decade, no single approach has emerged as a replacement to expression by in vitro transcription with T7 RNAP followed by denaturing PAGE and refolding. While these alternative techniques provide clear advantages over the classic RNA workflow, they have yet to prove as easy and reproducible as the classic workflow. Instead, many of these alternative methods are being used to meet specific challenges presented by an RNA or the experimental approach that preclude traditional methods of expression or purification. However, as these methodologies improved upon, it is likely that denaturing purification of RNA will become as infrequent as for proteins.
Highlights.
Denaturing RNA purification protocols can be unsuitable for various reasons
RNA can be natively expressed in E. coli using a stabilizing tRNA scaffold
A series of RNA affinity tags have been developed for native purification
Simple gel filtration and ion exchange protocols have been established for RNA
Acknowledgments
The author would like to thank Dr. Jennifer Pfingsten for critical feedback on the manuscript and Prof. Jeffrey Kieft for many discussions relating to the development of native purification methods. This work was supported by grants from the National Institutes of Health (GM 073850 and GM 083953).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serganov A, Patel DJ. Ribozymes, riboswitches and beyond: regulation of gene expression without proteins. Nat Rev Genet. 2007;8:776–790. doi: 10.1038/nrg2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards AL, Garst AD, Batey RT. Determining structures of RNA aptamers and riboswitches by X-ray crystallography. Methods Mol Biol. 2009;535:135–163. doi: 10.1007/978-1-59745-557-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milligan JF, Uhlenbeck OC. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- 4.Pleiss JA, Derrick ML, Uhlenbeck OC. T7 RNA polymerase produces 5′ end heterogeneity during in vitro transcription from certain templates. RNA. 1998;4:1313–1317. doi: 10.1017/s135583829800106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyes FE, Garst AD, Batey RT. Strategies in RNA crystallography. Methods Enzymol. 2009;469:119–139. doi: 10.1016/S0076-6879(09)69006-6. [DOI] [PubMed] [Google Scholar]
- 6.Uhlenbeck OC. Keeping RNA happy. RNA. 1995;1:4–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Kladwang W, Hum J, Das R. Ultraviolet shadowing of RNA can cause significant chemical damage in seconds. Sci Rep. 2012;2:517. doi: 10.1038/srep00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfeld M, Solomatin SV, Herschlag D. Removal of covalent heterogeneity reveals simple folding behavior for P4-P6 RNA. J Biol Chem. 2011;286:19872–19879. doi: 10.1074/jbc.M111.235465.. • A detailed analysis of damage caused by one step of denaturing polyacrylamide gel electrophoresis purification of RNA.
- 9.Lukavsky PJ, Puglisi JD. Large-scale preparation and purification of polyacrylamide-free RNA oligonucleotides. RNA. 2004;10:889–893. doi: 10.1261/rna.5264804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponchon L, Dardel F. Recombinant RNA technology: the tRNA scaffold. Nat Methods. 2007;4:571–576. doi: 10.1038/nmeth1058.. •• This describes the first practical and general method to stably express a variety of RNAs in E. coli.
- 12.Ponchon L, Beauvais G, Nonin-Lecomte S, Dardel F. A generic protocol for the expression and purification of recombinant RNA in Escherichia coli using a tRNA scaffold. Nat Protoc. 2009;4:947–959. doi: 10.1038/nprot.2009.67. [DOI] [PubMed] [Google Scholar]
- 13.Ponchon L, Catala M, Seijo B, El Khouri M, Dardel F, Nonin-Lecomte S, Tisne C. Co-expression of RNA-protein complexes in Escherichia coli and applications to RNA biology. Nucleic Acids Res. 2013;41:e150. doi: 10.1093/nar/gkt576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponchon L, Beauvais G, Nonin-Lecomte S, Dardel F. Selective RNase H cleavage of target RNAs from a tRNA scaffold. Methods Mol Biol. 2012;941:9–18. doi: 10.1007/978-1-62703-113-4_2. [DOI] [PubMed] [Google Scholar]
- 15.Nelissen FH, Leunissen EH, van de Laar L, Tessari M, Heus HA, Wijmenga SS. Fast production of homogeneous recombinant RNA--towards large-scale production of RNA. Nucleic Acids Res. 2012;40:e102. doi: 10.1093/nar/gks292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chui HM, Desaulniers JP, Scaringe SA, Chow CS. Synthesis of helix 69 of Escherichia coli 23S rRNA containing its natural modified nucleosides, m(3)Psi and Psi. J Org Chem. 2002;67:8847–8854. doi: 10.1021/jo026364m. [DOI] [PubMed] [Google Scholar]
- 17.Vendeix FA, Murphy FVt, Cantara WA, Leszczynska G, Gustilo EM, Sproat B, Malkiewicz A, Agris PF. Human tRNA(Lys3)(UUU) is pre-structured by natural modifications for cognate and wobble codon binding through keto-enol tautomerism. J Mol Biol. 2012;416:467–485. doi: 10.1016/j.jmb.2011.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paige JS, Nguyen-Duc T, Song W, Jaffrey SR. Fluorescence imaging of cellular metabolites with RNA. Science. 2012;335:1194. doi: 10.1126/science.1218298.. • Describes another use of the tRNA scaffold that enables the expression of RNA-based biosensors in bacteria.
- 19.Nakayama S, Luo Y, Zhou J, Dayie TK, Sintim HO. Nanomolar fluorescent detection of c di-GMP using a modular aptamer strategy. Chem Commun (Camb) 2012;48:9059–9061. doi: 10.1039/c2cc34379g. [DOI] [PubMed] [Google Scholar]
- 20.Kellenberger CA, Wilson SC, Sales-Lee J, Hammond MC. RNA-Based Fluorescent Biosensors for Live Cell Imaging of Second Messengers Cyclic di-GMP and Cyclic AMP-GMP. J Am Chem Soc. 2013;135:4906–4909. doi: 10.1021/ja311960g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song W, Strack RL, Jaffrey SR. Imaging bacterial protein expression using genetically encoded RNA sensors. Nat Methods. 2013;10:873–875. doi: 10.1038/nmeth.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieft JS, Batey RT. A general method for rapid and nondenaturing purification of RNAs. RNA. 2004;10:988–995. doi: 10.1261/rna.7040604.. • The first description of a native affinity RNA purification method and details the general considerations for the creation of such a method.
- 23.Aviv H, Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972;69:1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira MJ, Behera V, Walter NG. Nondenaturing purification of co-transcriptionally folded RNA avoids common folding heterogeneity. PLoS One. 2010;5:e12953. doi: 10.1371/journal.pone.0012953.. • Demonstrates that many of the conformational heterogeneity issues relating to the refolding of RNA from denaturing purification techniques can be circumvented using native approaches.
- 25.Srisawat C, Engelke DR. Streptavidin aptamers: affinity tags for the study of RNAs and ribonucleoproteins. RNA. 2001;7:632–641. doi: 10.1017/s135583820100245x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srisawat C, Goldstein IJ, Engelke DR. Sephadex-binding RNA ligands: rapid affinity purification of RNA from complex RNA mixtures. Nucleic Acids Res. 2001;29:E4. doi: 10.1093/nar/29.2.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker SC, Scott FH, Srisawat C, Engelke DR. RNA affinity tags for the rapid purification and investigation of RNAs and RNA-protein complexes. Methods Mol Biol. 2008;488:23–40. doi: 10.1007/978-1-60327-475-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butter F, Scheibe M, Morl M, Mann M. Unbiased RNA-protein interaction screen by quantitative proteomics. Proc Natl Acad Sci U S A. 2009;106:10626–10631. doi: 10.1073/pnas.0812099106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iioka H, Loiselle D, Haystead TA, Macara IG. Efficient detection of RNA-protein interactions using tethered RNAs. Nucleic Acids Res. 2011;39:e53. doi: 10.1093/nar/gkq1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemay V, Hossain A, Osheim YN, Beyer AL, Dragon F. Identification of novel proteins associated with yeast snR30 small nucleolar RNA. Nucleic Acids Res. 2011;39:9659–9670. doi: 10.1093/nar/gkr659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson HE, Liljas L, Uhlenbeck OC. RNA recognition by the MS2 phage coat protein. Seminars in Virology. 1997;8:176–185. [Google Scholar]
- 32.Coller J, Wickens M. Tethered function assays: An adaptable approach to study RNA regulatory proteins. Translation Initiation: Extract Systems and Molecular Genetics. 2007;429:299–321. doi: 10.1016/S0076-6879(07)29014-7. [DOI] [PubMed] [Google Scholar]
- 33.Jurica MS, Licklider LJ, Gygi SP, Grigorieff N, Moore MJ. Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. Rna-a Publication of the Rna Society. 2002;8:426–439. doi: 10.1017/s1355838202021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Said N, Rieder R, Hurwitz R, Deckert J, Urlaub H, Vogel J. In vivo expression and purification of aptamer-tagged small RNA regulators. Nucleic Acids Res. 2009;37 doi: 10.1093/nar/gkp719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Tomasso G, Lampron P, Dagenais P, Omichinski JG, Legault P. The ARiBo tag: a reliable tool for affinity purification of RNAs under native conditions. Nucleic Acids Res. 2011;39:e18. doi: 10.1093/nar/gkq1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HY, Haurwitz RE, Apffel A, Zhou K, Smart B, Wenger CD, Laderman S, Bruhn L, Doudna JA. RNA-protein analysis using a conditional CRISPR nuclease. Proc Natl Acad Sci U S A. 2013;110:5416–5421. doi: 10.1073/pnas.1302807110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batey RT, Kieft JS. Improved native affinity purification of RNA. RNA. 2007;13:1384–1389. doi: 10.1261/rna.528007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvail-Lacoste A, Di Tomasso G, Piette BL, Legault P. Affinity purification of T7 RNA transcripts with homogeneous ends using ARiBo and CRISPR tags. RNA. 2013;19:1003–1014. doi: 10.1261/rna.037432.112.. •• The only native affinity approach that can generate a chemically homogenous product through processing of the 5′- and 3′-ends during the purification protocol.
- 39.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 40.Huang F, Wang G, Coleman T, Li N. Synthesis of adenosine derivatives as transcription initiators and preparation of 5′ fluorescein- and biotin-labeled RNA through one-step in vitro transcription. RNA. 2003;9:1562–1570. doi: 10.1261/rna.5106403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang F, He J, Zhang Y, Guo Y. Synthesis of biotin-AMP conjugate for 5′ biotin labeling of RNA through one-step in vitro transcription. Nat Protoc. 2008;3:1848–1861. doi: 10.1038/nprot.2008.185. [DOI] [PubMed] [Google Scholar]
- 42.Kirby R, Cho EJ, Gehrke B, Bayer T, Park YS, Neikirk DP, McDevitt JT, Ellington AD. Aptamer-based sensor arrays for the detection and quantitation of proteins. Anal Chem. 2004;76:4066–4075. doi: 10.1021/ac049858n. [DOI] [PubMed] [Google Scholar]
- 43.Collett JR, Cho EJ, Lee JF, Levy M, Hood AJ, Wan C, Ellington AD. Functional RNA microarrays for high-throughput screening of antiprotein aptamers. Anal Biochem. 2005;338:113–123. doi: 10.1016/j.ab.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Coleman TM, Wang G, Huang F. Superior 5′ homogeneity of RNA from ATP-initiated transcription under the T7 phi 2.5 promoter. Nucleic Acids Res. 2004;32:e14. doi: 10.1093/nar/gnh007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn JJ, Studier FW. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983;166:477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- 46.Luo Y, Eldho NV, Sintim HO, Dayie TK. RNAs synthesized using photocleavable biotinylated nucleotides have dramatically improved catalytic efficiency. Nucleic Acids Res. 2011;39:8559–8571. doi: 10.1093/nar/gkr464.. • Alternative affinity purification technique that uses a 5′ photocleavable biotin tag incorporated during transcription.
- 47.Kao C, Rudisser S, Zheng M. A simple and efficient method to transcribe RNAs with reduced 3′ heterogeneity. Methods. 2001;23:201–205. doi: 10.1006/meth.2000.1131. [DOI] [PubMed] [Google Scholar]
- 48.Kao C, Zheng M, Rudisser S. A simple and efficient method to reduce nontemplated nucleotide addition at the 3 terminus of RNAs transcribed by T7 RNA polymerase. RNA. 1999;5:1268–1272. doi: 10.1017/s1355838299991033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim I, McKenna SA, Viani Puglisi E, Puglisi JD. Rapid purification of RNAs using fast performance liquid chromatography (FPLC) RNA. 2007;13:289–294. doi: 10.1261/rna.342607.. • Describes a detailed protocol for purification of RNA using gel filtration chromatography.
- 50.McKenna SA, Kim I, Puglisi EV, Lindhout DA, Aitken CE, Marshall RA, Puglisi JD. Purification and characterization of transcribed RNAs using gel filtration chromatography. Nat Protoc. 2007;2:3270–3277. doi: 10.1038/nprot.2007.480. [DOI] [PubMed] [Google Scholar]
- 51.Easton LE, Shibata Y, Lukavsky PJ. Rapid, nondenaturing RNA purification using weak anion-exchange fast performance liquid chromatography. RNA. 2010;16:647–653. doi: 10.1261/rna.1862210.. •• This article describes a method for purifying RNAs by FPLC using a DEAE anion exchange chromatography.
- 52.Keel AY, Easton LE, Lukavsky PJ, Kieft JS. Large-scale native preparation of in vitro transcribed RNA. Methods Enzymol. 2009;469:3–25. doi: 10.1016/S0076-6879(09)69001-7. [DOI] [PubMed] [Google Scholar]
- 53.Sosnick TR, Pan T. RNA folding: models and perspectives. Curr Opin Struct Biol. 2003;13:309–316. doi: 10.1016/s0959-440x(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 54.Novikova IV, Hennelly SP, Sanbonmatsu KY. Structural architecture of the human long non-coding RNA, steroid receptor RNA activator. Nucleic Acids Res. 2012;40:5034–5051. doi: 10.1093/nar/gks071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xayaphoummine A, Viasnoff V, Harlepp S, Isambert H. Encoding folding paths of RNA switches. Nucleic Acids Res. 2007;35:614–622. doi: 10.1093/nar/gkl1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shajani Z, Sykes MT, Williamson JR. Assembly of bacterial ribosomes. Annu Rev Biochem. 2011;80:501–526. doi: 10.1146/annurev-biochem-062608-160432. [DOI] [PubMed] [Google Scholar]
- 57.Vogel U, Jensen KF. The RNA chain elongation rate in Escherichia coli depends on the growth rate. J Bacteriol. 1994;176:2807–2813. doi: 10.1128/jb.176.10.2807-2813.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol. 2007;14:796–806. doi: 10.1038/nsmb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makarova OV, Makarov EM, Sousa R, Dreyfus M. Transcribing of Escherichia coli genes with mutant T7 RNA polymerases: stability of lacZ mRNA inversely correlates with polymerase speed. Proc Natl Acad Sci U S A. 1995;92:12250–12254. doi: 10.1073/pnas.92.26.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonner G, Patra D, Lafer EM, Sousa R. Mutations in T7 RNA polymerase that support the proposal for a common polymerase active site structure. EMBO J. 1992;11:3767–3775. doi: 10.1002/j.1460-2075.1992.tb05462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonner G, Lafer EM, Sousa R. Characterization of a set of T7 RNA polymerase active site mutants. J Biol Chem. 1994;269:25120–25128. [PubMed] [Google Scholar]
- 62.Toor N, Keating KS, Taylor SD, Pyle AM. Crystal structure of a self-spliced group II intron. Science. 2008;320:77–82. doi: 10.1126/science.1153803.. •• An outstanding example of the critical application of native RNA purification techniques to the structure determination of a large RNA.
- 63.Swisher JF, Su LJ, Brenowitz M, Anderson VE, Pyle AM. Productive folding to the native state by a group II intron ribozyme. J Mol Biol. 2002;315:297–310. doi: 10.1006/jmbi.2001.5233. [DOI] [PubMed] [Google Scholar]
- 64.Kieft JS, Costantino DA, Filbin ME, Hammond J, Pfingsten JS. Structural methods for studying IRES function. Methods Enzymol. 2007;430:333–371. doi: 10.1016/S0076-6879(07)30013-X. [DOI] [PubMed] [Google Scholar]