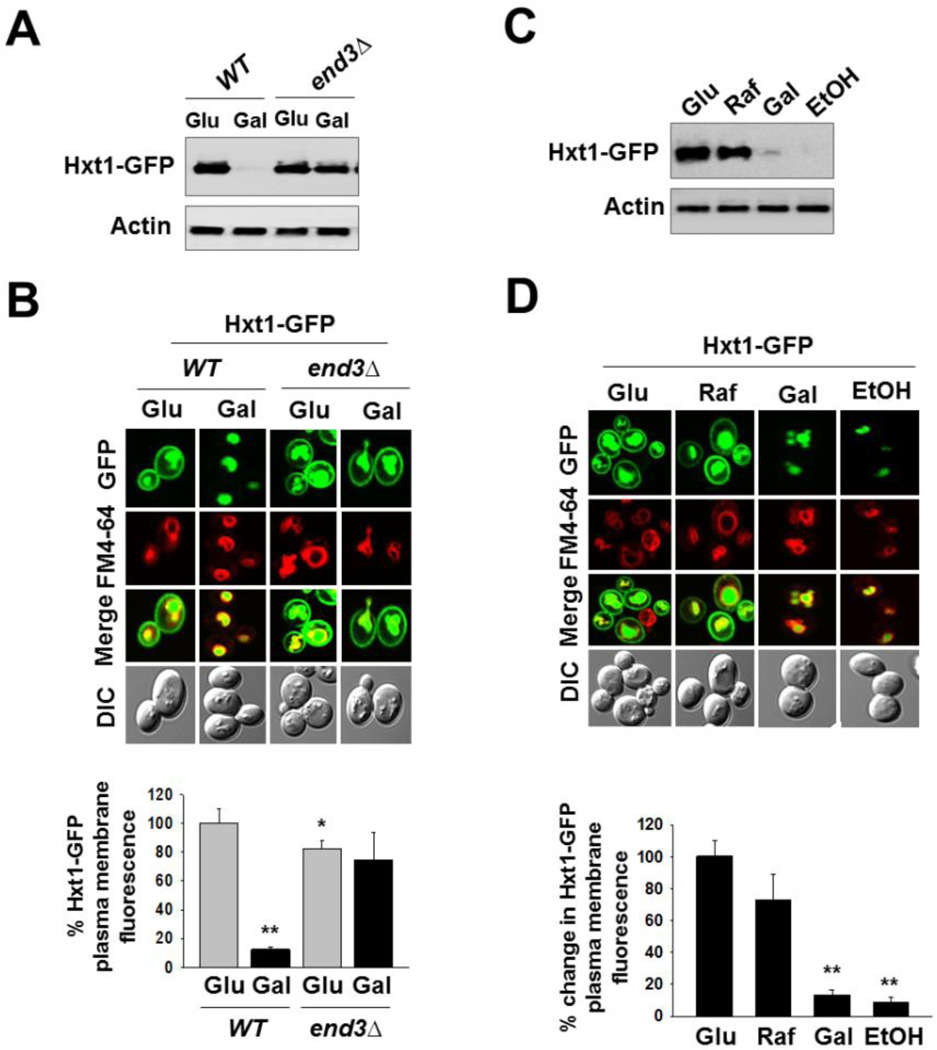
Fig. 2.
Glucose starvation induces endocytosis and subsequent degradation of Hxt1. (A) Yeast cells (WT and end3Δ) expressing Hxt1-GFP were grown in SC-2% glucose (Glu) medium to mid-log phase and shifted to SC medium containing 2% galactose (Gal) for 6 h. Membrane-enriched fractions were immunoblotted with anti-GFP antibody. (B) Yeast cells (WT and end3Δ) expressing Hxt1-GFP were grown as described in (A). Confocal microscope image (top panel) and quantification of relative GFP fluorescence in the plasma membrane (bottom panel, *P < 0.05, **P < 0.001) were shown. (C) Yeast cells (WT) expressing Hxt1-GFP were grown in SC-2% glucose (Glu) medium to mid-log phase and shifted to SC medium containing either 2% raffinose (Raf), 2% galactose (Gal) or 2% ethanol (EtOH) for 6 h. Membrane fractions were immunoblotted with anti-GFP antibody. (D) Yeast cells (WT) expressing Hxt1-GFP were grown as described in (C). Confocal microscope image (top panel) and quantification of relative GFP fluorescence in the plasma membrane (bottom panel, **P < 0.001) were shown. The FM6-64 dye was used to stain the vacuolar membrane (red), and actin was served as loading control in (A) and (C). Pma1 is frequently used as a loading control for membrane fractions; it, however, is not appropriate for this study because its expression is critically regulated by glucose [52].