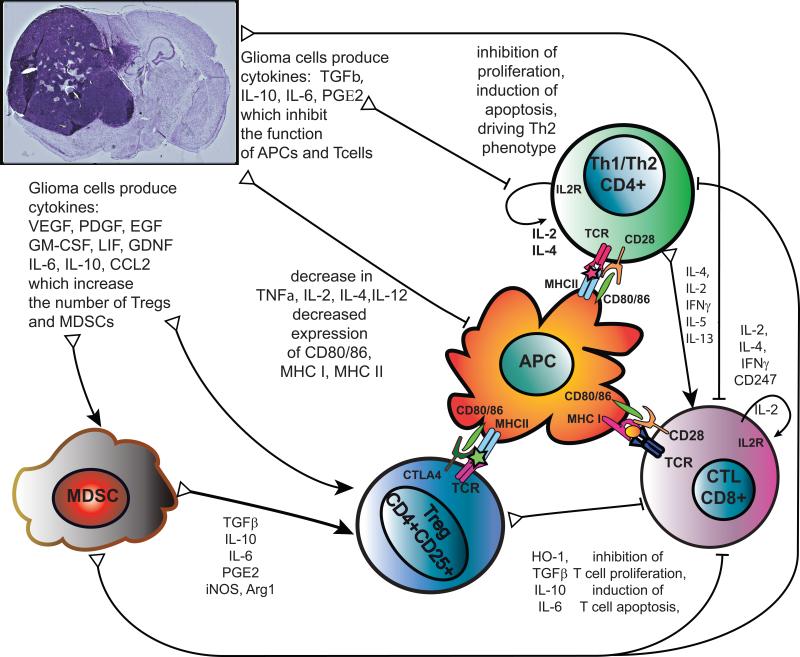
Figure 4. Mechanisms of tumor-induced immune suppression.
Glioma cells inhibit T cell function through cytokines that act directly on T cells, like: TGFβ, IL-10, IL-6, which inhibit T cell production of IL-2, IL-4, IFN-γ, expression of IL2R and CD247, signaling downstream of the T cell receptor and activation of CTLs by T helper cells. This results in inhibition of T cell activation and proliferation. Through enhanced production of reactive oxygen species by PGE2, glioblastomas also directly induce T cell apoptosis. IL-2, IL-4, IFN-γ can be used to stimulate T cell function and aid anti-glioma therapies. Glioma cells can also inhibit T cell function indirectly by impairing differentiation, maturation, activation and function of antigen presenting cells (APC), reducing the expression of co-stimulatory molecules (CD80/CD86), MHC-I and MHC-II, decreasing the production of immunostimulatory cytokines TNFα, IL-2, IL-4, IL-12. Therapies to increase production of CD80/86 and immunostimulatory molecules show promise as adjuvant therapies for glioma. In addition, glioma cells produce cytokines (VEGF, PDGF, LIF, GDNF, IL-6, IL-10, CCL2) which alter hematopoietic lineages to promote differentiation towards immunosuppressive phenotypes and increase the number of Myeloid Derived Suppressor Cells (MDSCs) and regulatory T cells (Tregs). These are recruited to the tumor microenvironment and circulate back to lymphoid organs to suppress anti-tumor immune responses further. Abbreviations used: Arg1, arginase 1; CCL2, Chemokine ligand 2 (also known as monocyte chemotactic protein -1 MCP-1); EGF, epidermal growth factor; GDNF, glial derived neurotrophic factor; GM-CSF, granulocyte macrophage colony stimulating factor; HO-1 heme oxygenase 1; iNOS, inducible nitric oxide synthase (also known as NOS2); LIF, leukemia inhibitory factor; PGE2, Prostaglandin E2; PDGF, platelet-derived growth factor; TGFβ, Transforming growth factor beta; TNFα, Tumor necrosis factor alpha, VEGF, vascular endothelial growth factor.