Figure 2. Hypotheses for why BnAb+ B-cells acquire unusually high levels of VDJ mutations during chronic HIV-1 infection.
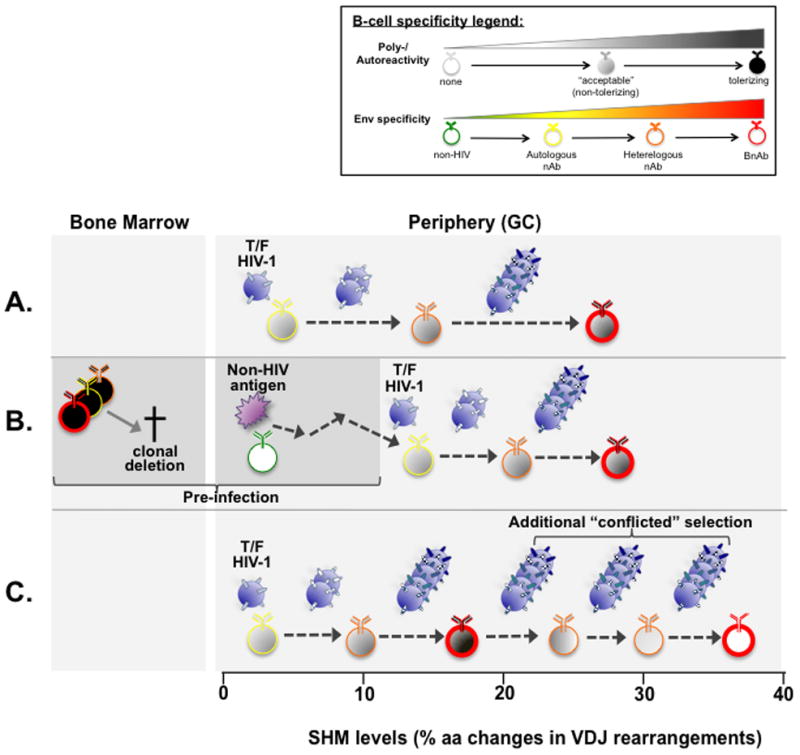
In a standard (non-tolerance) model (A), GC B cells with no/minimal reactivity to mature Envs (57••,65,66) interact with founder/transmitted Envs and undergo extensive AM, driven by viral escape/Env diversification pressure (21••), which demands difficult structural modifications to achieve both FRW flexibility and Env affinity (67•). Note that as shown, B-cells with “acceptable” (non-tolerizing) polyeractivity prior to entering the GC reaction may be selected for their ability to heteroligate Env (68), but poly-/self-reactivity may also be acquired during AM, as in the CH103-CH106 BnAb lineage (21••). In a model where self-tolerance indirectly impacts excess SHM (52••,57••) (B), B-cells with BnAb reactivity that mimic host antigens are clonally deleted in BM development, creating “holes in the repertoire”. In the absence of competition, weakly cross-reactive clones that already underwent non-Env driven AM can then participate in additional, Env-driven AM. Finally, in a model where self-tolerance directly drives excess SHM (69••)(C), B-cells that acquire both self and BnAb specificity via AM use additional SHM/selection to retain BnAb specificity, while removing tolerizing self-reactivity. Such a process would assume close, but not complete BnAb mimicry of self-epitopes. Thus, the degree of additional selection/SHM would be proportional to how closely a particular BnAb epitope mimics self-antigen(s). Note that these hypotheses are not mutually exclusive: for example, the “tolerance-based” models shown in (B–C) are shown in combination with the “standard” model, depicted in (A).
