Fig. 6.
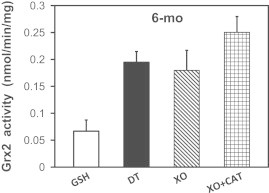
Modulation of Grx2 activity by ROS produced by the xanthine oxidase system in vitro. Isolated heart mitochondria from young adult rats were lysed in the presence of either GSH (1 mM) to maintain the inactive Grx2 complex (white bar), or sodium dithionite (10 mM, DT) to fully dissociate Grx2 (black bar). Mitochondrial samples lysed in the presence of GSH were then combined with xanthine oxidase (XO, 10 μM) to generate superoxide radicals and/or hydrogen peroxide upon metabolizing xanthine. Also added were either vehicle (diagonal bar), or catalase (checkered bar; CAT, 1 mg/mL) to selectively scavenge H2O2. The reactions were initiated by adding xanthine (2 mM), and then Grx2 activity was determined for each sample, with results reported as nmol/min/mg of mitochondrial protein (mean±SEM, n=6).
