Table 1.
Proof-of-concept and reaction optimization experiments
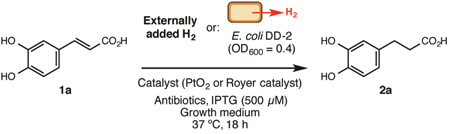 | ||||
|---|---|---|---|---|
| Entry | Growth medium | Cells/H2 added[a] |
Catalyst, mol% | Conversion (%)[b] |
| 1 | LB + glucose | no/yes | PtO2, 40 mol% | 15 |
| 2 | M9 glucose | no/yes | PtO2, 40 mol% | 100 |
| 3 | LB + glucose | yes/yes | PtO2, 40 mol% | 6 |
| 4 | M9 glucose | yes/yes | PtO2, 40 mol% | 91 |
| 5 | LB + glucose | yes/no | PtO2, 40 mol% | 0 |
| 6 | M9 glucose | yes/no | PtO2, 40 mol% | 15 |
| 7 | M9CA glucose + Fe[c] | yes/no | PtO2, 20 mol% | 56 |
| 8 | M9CA glucose + Fe | yes/no | Royer, 8 mol%[d] | 100 |
| 9 | M9CA glucose + Fe | yes/no | Royer, 8 mol% | 100/87[e] |
Reactions were performed at a 5 mM substrate concentration in 5 mL of growth medium containing ampicillin (50 µg/mL), spectinomycin (25 µg/mL), chloramphenicol (12.5 µg/mL), and IPTG (500 µM) under an atmosphere of either hydrogen or nitrogen in 16 mL Hungate tubes with shaking at 190 rpm.
E. coli strain DD-2 was used, OD600 = 0.4.
Determined by 1H NMR.
M9CA glucose + Fe medium contains Fe(NH4)2(SO4)2 (50 µM) and casamino acids (5 g/L).
Royer catalyst is 2.44 wt% palladium on polyethyleneimine/silica gel.
Reaction was performed on a 9 mmol scale (1.6 g of 1a) with 8 mol% Royer catalyst at a substrate concentration of 10 mM for 48 h (87% isolated yield).
