Abstract
Objectives:
Activation of mineralization process in periradicular tissues following the injuries, is important in repair mechanisms. The objective of this study was to investigate the effects of CEM cement on survival and mineralization of human mesenchymal stem cells (hMSCs) and compare it with MTA.
Materials and Methods:
hMSCs that were planted on test material extracts and culture media were the experimental and control groups, respectively. The cytotoxicity of these materials was investigated using Methyl thiazol tetrazolium assay. After 7 days, alizarin red staining, alkaline phosphatase (ALP) assays, and qRT-PCR were used to assess the mineralization, expression of ALP, and gene expression (collagen type 1 and osteocalcin), respectively. The results were evaluated by ANOVA analysis and multiple comparisons test. P < 0.05 was considered as statistically significant.
Results:
Cell viability was not significantly different. Alizarin red and alkaline phosphatase staining showed mineralization in all three groups. In qRT-PCR, the expression of collagen type 1 is not significantly different among the three groups. Osteocalcin gene expression was significantly higher in the CEM group compared to the control (P < 0.05).
Conclusion:
CEM cement has acceptable toxicity and could induce mineralization process and enhance osteocalcin gene expression which is associated with mineralization in hMSCs.
Keywords: CEM cement, human mesenchymal stem cell, mineralization, MTA, osteoblastic differentiation
INTRODUCTION
Root-end filling materials should ideally be biocompatible, insoluble, induce periapical tissue regeneration, have effective sealing ability, as well as dimensional stability, moisture imperviousness, radiopacity, nontoxicity, non-carcinogenicity, and ease of handling.[1,2]
Mineral trioxide aggregate (MTA) is an endodontic material with acceptable biocompatibility and osteoconductive properties.[3] It has a high success rate when used to seal the perforation area and retrograde cavities in periradicular surgery.[4,5] High cost, long setting time, and potential for discoloration are the main drawbacks of this material.[5,6] Furthermore the antibacterial properties of MTA are unpredictable, and handling characteristics are not ideal.[6]
As a mean to address these shortcomings, a new biomaterial was developed called calcium-enriched mixture (CEM) cement using different calcium compounds.[7]
CEM cement has a good sealing ability when used as a root-end filling material.[8] It has also shown favorable biological response as a pulp-capping agent.[9] The other advantages of this biomaterial are the acceptable physical properties such as shorter setting time (less than 1 hour), greater flow characteristics, and less film thickness when compared to MTA.[9] In addition, it has been shown that CEM cement has the ability to induce hydroxyapatite formation over its surface in normal saline solution.[10]
Hard tissue formation over the root-end filling material requires the process of cell differentiation into hard tissue-forming cells and activation of the mineralization process.[11] Mesenchymal stem cells play an important role in tissue and bone remodeling,[12] and differentiation of mesenchyme-derived stem cells are thought to be affected by the local environmental factors.[12,13]
Therefore, the objective of this study was to investigate the effects of CEM cement on survival and mineralization of human mesenchymal stem cells and compare it with ProRoot MTA.
MATERIALS AND METHODS
Cell culture
Human bone marrow-derived mesenchymal stem cells (hMSCs) were used for this study. Cells were grown in Dulbecco modified Eagle medium (DMEM; Gibco, Germany) supplemented with 2 mmol/L L-glutamine (Gibco, Germany), 10% fetal bovine serum (FBS; Gibco, Germany), 100 U/mL of penicillin, and 100 μg/ml of streptomycin. Cell cultures were maintained at 37°C, 95% humidity, and 5% CO2 . Cells used in these experiments were between passages 3 and 7.
Material preparation
Tested materials were ProRoot White MTA (Dentsply Endodontics, Tulsa, OK) and CEM cement (BioniqueDent, Tehran, Iran). These materials were prepared following the manufacturer's instructions under sterile conditions. A liquid/powder ratio of 0.3 mL/g was used for all experiment groups. Extract of materials were made as follows: Disc-shaped specimens (3-mm diameter × 1-mm thick) of each material were prepared then these specimens were placed individually in one of the wells of sterile 24-well plates. The materials were maintained at 37o C and 95% humidity for 4 hours. Afterward, 1 mL of DMEM was added to each well, and incubated at 37°C and 95% relative humidity for the duration of 24 hours. Aliquots were then extracted and cells were treated every 3 days with this solution.
Three wells for each test and control group were used; control groups were either plain DMEM or osteogenic medium.
Cell Inoculation
Trypsinized cells were seeded on 96-well plates for Methyl thiazol tetrazolium (MTT) assay at a density of 2 × 103 cells per well in 200 μL of growth medium. Afterward, cells were incubated for 24 hours at 37°C, 5% CO2 and 95% relative humidity, After 24 hours of incubation, 200 μL aliquots of the test extracts and control media (DMEM plain), were collected for MTT assay to assess the cytotoxicity of test materials.
Surface-treated 24-well plates were used for mineralization tests (Alizarin Red Staining, Alkaline phosphatase assay and qPCR). Cells were planted at a density of 2 × 104 cells per well in 200 μL of growth medium. After 48 hours, cells were switched to osteogenic media containing minimal essential medium alpha Eagle (α -MEM), β-glycerolphsphate [10 mmol/L], ascorbate-2-phosphate [0.2 mmol/L], dexamethasone [100 mmol/L], and 15% FBS. Cells were treated every 3 days with freshly prepared culture medium containing osteogenic media and aliquots of test extracts. Cells plated in osteogenic media plain were considered as control group.
MTT Experiment
Cell viability and proliferation were studied using an MTT Cell Proliferation Kit (Roche Applied Science, Indianapolis, IN). Briefly, cells were incubated at 37°C, 95% humidity, and 5% Co2 for 24 hours after cell inoculation as explained above. A 20 μL volume of MTT working solution was added to each well. Subsequently, the mixture was incubated for another 4 hours. Each well then was washed using 200 μL of phosphate-buffered saline. Afterward, 200 μL of dimethyl sulfoxide was added to each well. The plates were centrifuged at 1000 RPM for 5 minutes. Optical density (OD) at 492 nm was measured using an Eliza reader (Sunrise, Tecan).
Alizarin red staining
Mineralization was evaluated by alizarin red staining (Sigma-Aldrich Co.). The cells were seeded in the 24-well plates containing osteogenic media and cell extracts afterwards Cells were incubated at 37°C, 5% Co2, and 95% humidity for 7 days. Mediums were replaced every 3 days. On the 7th day the cells were stained with Alizarin red solution and observed with microscope.
Alkaline phosphatase assay
Cells were seeded in the 24-well plates. After 7 days incubation at 37°C, 5% CO2, and 95% humidity, Alkaline Phosphatase Detection Kit (MILLIPORE) was used to assess the expression of ALP, a typical osteoblast marker. Cells were observed under the microscope to see the red stains. These stains reveal the presence of alkaline phosphatase.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
The cells which were seeded in the 24-well plates containing osteogenic media plus test extracts that incubated for 7 days were used to perform quantitative RT-PCR. Three wells of each test material and control groups were used in the analysis. Trizol Reagent (Invitrogen, Carlsbad, CA) was used to homogenize the cells. The mixture was centrifuged at 12000g at 4°C for 15 minutes. The RNase-free water was used to dissolve the RNA and it was stored at −80°C. Complementary DNA was generated from the extracted RNA using RT-PCR with a PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). The expression of two specific marker genes (collagen type I (col1) and osteocalcin (ocn)) was determined using specific primers: collagen type I (Sense primer: 5’-GGAAATGCTGGACCCCCTGGCCC-3’, Antisense primer: 5’-AGCAGGACCATCAGCACCAGGGG-3’) and osteocalcin (Sense primer: 5’-GCTACCTGTATCAATGGCTG-3’, Antisense primer: 5’-GACCGGGCCGTATAGGCC-3’).
Expression of RNA was normalized using Glyceraldehyde 3- phosphate dehydrogenase (GAPDH) gene.
Statistical analysis
ANOVA analysis and multiple comparisons test (Tamhane) were used via SPSS software 19 (SPSS, Chicago, IL, USA). P < 0.05 was considered as statistically significant.
RESULTS
MTT experiment analysis
Although cell viability and proliferation in the MTA group was less than those of CEM cement and the control groups, no statistical difference was observed among the groups. (P-value ≥0.05)
Alizarin red staining
Alizarin red staining nodules were observed in all the groups (MTA, CEM cement, and control), mineralization nodule formation in the CEM cement group was the most prominent [Figure 1].
Figure 1.
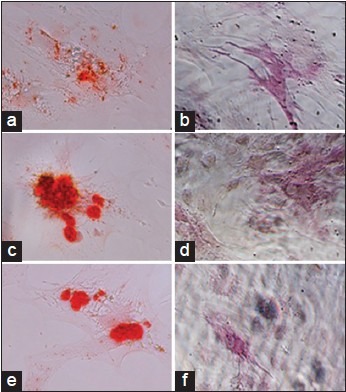
Micrographs of hMSCs after 7 days. (A-C: Alizarin red staining of specimens and D-F: Expression of ALP in the samples) Mineralization nodule formations were observed in the group of MTA (a), CEM cement (b), and Control (c). Furthermore, expression of ALP was detected in all three groups (MTA (d), CEM cement (e), and control (f))
Alkaline phosphatase assay
Expression of ALP was detected in all three groups (MTA, CEM cement, and control), but the amount of stained areas which would indicate the presence of ALP was not different in these groups [Figure 1].
Gene expression analysis
Col1. After 7 days, the difference of col1 expression level among all three groups (MTA, CEM cement, and control) was not significant [Figure 2 and Table 1].
Figure 2.
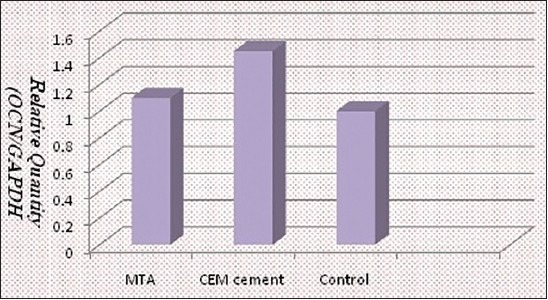
The relative expression of the Ocn gene normalized against a housekeeping gene (GAPDH)
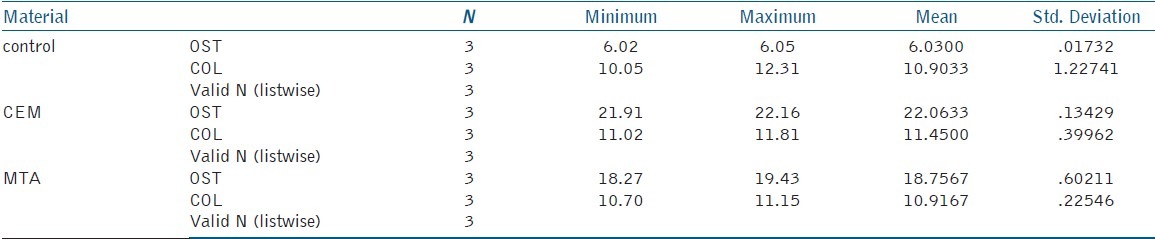
Table 1.
Mean and standard deviation of experimental groups in PCR analysis
Ocn. Ocn expression level in the CEM cement group was significantly more than the control group (P < 0.05). But the level of Ocn expression in the MTA group was not significantly different from the control group [Figure 2 and Tables 1 and 2].
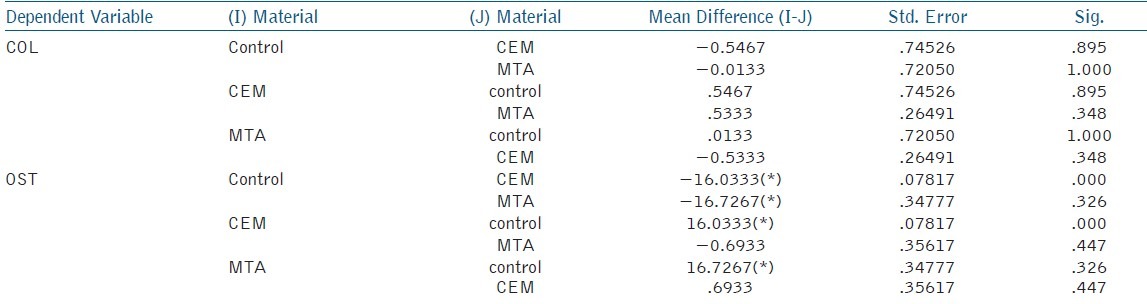
Table 2.
Mean and standard deviation for inter group comparisons in PCR analysis. The mean difference is significant at the 0.05 level
DISCUSSION
In this study the effect of biomaterials on mesenchymal stem cells was investigated using MTT assay, alkaline phosphatase assay, Alizarin red staining, and qRT-PCR. A comprehensive evaluation was performed to assess the survival and differentiation of hMSCs when placed adjacent to different biomaterials. To evaluate the gene expression level, qRT-PCR was used. Due to the exquisite sensitivity and accurate gene quantification of this method, qRT-PCR is considered the gold standard in the field of gene detection; and the use of this procedure has supplanted other approaches (e.g., Northern blotting, Southern blotting, and RNase protection assays).[14]
In the present study the ability of MTA and CEM cement to enhance tissue regeneration was evaluated; these biomaterials are commonly used in clinical practice such as root perforation, resorption repair, apexification, and apical surgery.[9,10,15,16,17,18]
The healing of injured tissue in the peridontium is clearly dependent on the biocompatibility of the repair material and cells interaction with them.[19] It has been demonstrated that the migration and differentiation of multipotent mesenchymal stem cells during the bone and periodontal healing and regeneration process are necessary.[20] In our study, hMSCs have been used as a model of multipotent mesenchymal stem cells to assess the effect of CEM cement and MTA on them because these cells are wildly involved in the wound-healing process of the alveolar bone.[19,20]
The results of this study showed that there is no statistical difference in cell survival among the MTA group, CEM cement group and control group. The obtained result was in accordance with previous studies.[5,19,21] We performed the MTT experiment as an adjunction to other methods of research for differentiation process. Only 24 hours period of cell viability was investigated because other time spans have been studied in other studies. MTA and CEM cement are primarily composed of different calcium salts, and it has been shown that calcium ions are released from hydrated MTA during the mixing of MTA powder and water.[20] In addition, it has been demonstrated that CEM cement causes the same bioactive reaction as MTA,[10] and the biocompatibility of these materials may be attributed to this fundamental bioactive reaction.
In this study no differences were observed among the groups when the ALP activity of the cells was being compared. This means that MTA and CEM cement did not significantly change the ALP activity of the cells; and it was in agreement with the results of Modareszadeh et al. study in MTA.[22]
The results of the Alizarin red staining experiment show that mineralization nodule formations in the group of CEM cement were more noticeable than those of other groups.
Due to the qualitative nature of these experimentations, confirmation of their results, using an accurate quantitative technique, seemed necessary. Therefore, qRT-PCR method was used to evaluate the mineralization-related gene expression in hMSCs. The expression of two specific marker genes col1 and ocn was determined. Col1 and ocn are important proteins in mineralized connective tissue and the increased gene expression of these proteins indicate enhanced mineralization process.[23,24,25] Zbenglin Yuan, et al.[23] evaluated the effect of MTA and bioaggregate on mineral-associated gene expression including col1 and ocn. In their study, MTA caused no significant enhancement in these two gene expressions which is in agreement with the current study. But CEM cement was able to induce the ocn of hMSCs,. The fact that ocn expression in the CEM cement group was significantly more than the control group; while MTA did not cause any significant change may be attributed to the differences in the amount of the released ions in the first day in the two aggregates. Therefore, pharmacokinetic studies on these two materials would be beneficial. Using human bone marrow-derived mesenchymal stem cells was an innovative approach for studying the effects of bioactive materials in endodontics which could possibly be more similar to real human body mechanisms of actions.
CONCLUSIONS
Within the limitations of the present in vitro study, it can be concluded that CEM cement has acceptable toxicity for clinical use and is able to induce mineralization process and enhance osteocalcin (OCN) gene expression which is associated with mineralization in hMSCs.
ACKNOWLEDGEMENT
This work was supported by the Iranian Center for Endodontic Research. The authors are indebted to Dr. M. Salehi and Dr. M. Bandehpour for the provision of laboratory facilities in the Cellular and Molecular Biology Research Center of Shahid Beheshti University of Medical Sciences. Based on a thesis submitted to the graduate faculty, Shahid Beheshti University of Medical science, in partial fulfillment of the requirements for the doctorate degree.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bodrumlu E. Biocompatibility of retrograde root filling materials: A review. Aust Endod J. 2008;34:30–5. doi: 10.1111/j.1747-4477.2007.00085.x. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–5. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 3.Tani-Ishii N, Hamada N, Watanabe K, Tujimoto Y, Teranaka T, Umemoto T. Expression of bone extracellular matrix proteins on osteoblast cells in the presence of mineral trioxide. J Endod. 2007;33:836–9. doi: 10.1016/j.joen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Tabarsi B, Parirokh M, Eghbal MJ, Haghdoost AA, Torabzadeh H, Asgary S. A comparative study of dental pulp response to several pulpotomy agents. Int Endod J. 2010;43:565–71. doi: 10.1111/j.1365-2591.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 5.Torabinejad M, Parirokh M. Mineral trioxide aggregate: A comprehensive literature review — part II: Leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005;31:665–8. doi: 10.1097/01.don.0000157993.89164.be. [DOI] [PubMed] [Google Scholar]
- 7.Asgary S, Eghbal MJ, Parirokh M. Sealing ability of a novel endodontic cement as a root-end filling material. J Biomed Mater Res A. 2008;87:706–9. doi: 10.1002/jbm.a.31678. [DOI] [PubMed] [Google Scholar]
- 8.Asgary S, Eghbal MJ, Parirokh M, Torabzadeh H. Sealing ability of three commercial mineral trioxide aggregates and an experimental root-end filling material. Iran Endod J. 2006;1:101–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:609–14. doi: 10.1016/j.tripleo.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009;35:147–52. doi: 10.1111/j.1747-4477.2008.00137.x. [DOI] [PubMed] [Google Scholar]
- 11.Economides N, Pantelidou O, Kokkas A, Tziafas D. Short-term periradicular tissue response to mineral trioxide aggregate (MTA) as root-end filling material. Int Endod J. 2003;36:44–8. doi: 10.1046/j.0143-2885.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- 12.Mosca JD, Hendricks JK, Buyaner D, Davis-Sproul J, Chuang LC, Majumdar MK, et al. Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res. 2000;379(Suppl):S71–90. doi: 10.1097/00003086-200010001-00011. [DOI] [PubMed] [Google Scholar]
- 13.Srisuwan T, Tilkorn DJ, Wilson JL, Morrison WA, Messer HM, Thompson EW, et al. Molecular aspects of tissue engineering in the dental field. Periodontol 2000. 2006;41:88–108. doi: 10.1111/j.1600-0757.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 14.Vanguilder HD, Vrana KE, Freeman WM. Twenty five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–26. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 15.Nosrat A, Asgary S, Eghbal MJ, Ghoddusi J, Bayat-Movahed S. Calcium enriched mixture cement as artificial apical barrier: A case series. J Conserv Dent. 2011;14:427–31. doi: 10.4103/0972-0707.87218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliani V, Baccetti T, Pace R, Pagavino G. The use of MTA in teeth with necrotic pulps and open apices. Dent Traumatol. 2002;18:217–21. doi: 10.1034/j.1600-9657.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi M, Shimizu A, Ebisu S. MTA for obturation of mandibular central incisors with open apices: Case report. J Endod. 2004;30:120–2. doi: 10.1097/00004770-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 18.von Arx T, Hänni S, Jensen SS. Clinical results with two different methods of root-end preparation and filling in apical surgery: Mineral trioxide aggregate and adhesive resin composite. J Endod. 2010;36:1122–9. doi: 10.1016/j.joen.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 19.D’Antò V, Di Caprio MP, Ametrano G, Simeone M, Rengo S, Spagnuolo G. Effect of mineral trioxide aggregate on mesenchymal stem cells. J Endod. 2010;36:1839–43. doi: 10.1016/j.joen.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–66. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Ghoddusi J, Tavakkol Afshari J, Donyavi Z, Brook A, Disfani R, Esmaeelzadeh M. Cytotoxic effect of a new endodontic cement and mineral trioxide aggregate on L929 line culture. Iran Endod J. 2008;3:17–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Modareszadeh MR, Di Fiore PM, Tipton DA, Salamat N. Cytotoxicity and alkaline phosphatase activity evaluation of endosequence root repair material. J Endod. 2012;38:1101–5. doi: 10.1016/j.joen.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Z, Peng B, Jiang H, Bian Z, Yan P. Effect of bioaggregate on mineral-associated gene expression in osteoblast cells. J Endod. 2010;36:1145–8. doi: 10.1016/j.joen.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama A, Ogiso B, Tanabe N, Takeichi O, Matsuzaka K, Inoue T. Behaviour of bone marrow osteoblast-like cells on mineral trioxide aggregate: Morphology and expression of type I collagen and bone-related protein mRNAs. Int Endod J. 2005;38:203–10. doi: 10.1111/j.1365-2591.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 25.Hakki SS, Bozkurt SB, Hakki EE, Belli S. Effects of mineral trioxide aggregate on cell survival, gene expression associated with mineralized tissues, and biomineralization of cementoblasts. J Endod. 2009;35:513–9. doi: 10.1016/j.joen.2008.12.016. [DOI] [PubMed] [Google Scholar]


