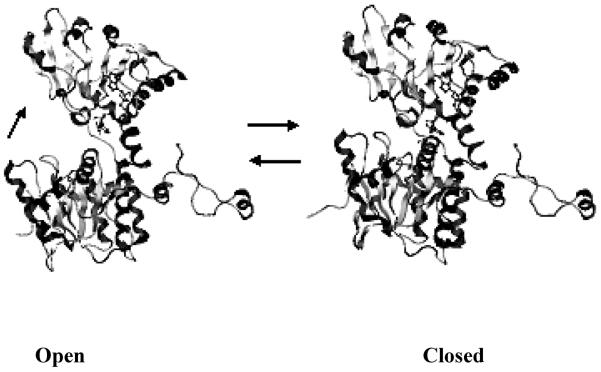
Figure 2.
Open conformation (pdb: 1KY4, rat source) and closed conformation (pdb: 1LI4, human source) of the SAHH monomer (created by Molecular Operation Environment). The top domain is the cofactor-binding domain; the bottom domain is the substrate-binding domain; the loop at the right is the C-terminal extension. SAHH in the open conformation contains cofactor NAD+ (ball-and-stick) and SAHH in closed conformation contains cofactor NADH (ball-and-stick) and oxidized inhibitor-NepA (ball-and-stick). From the open conformation, the substrate-binding domain rotates ~ 19° toward to the cofactor binding domain to form an active site for catalysis in the closed conformation.