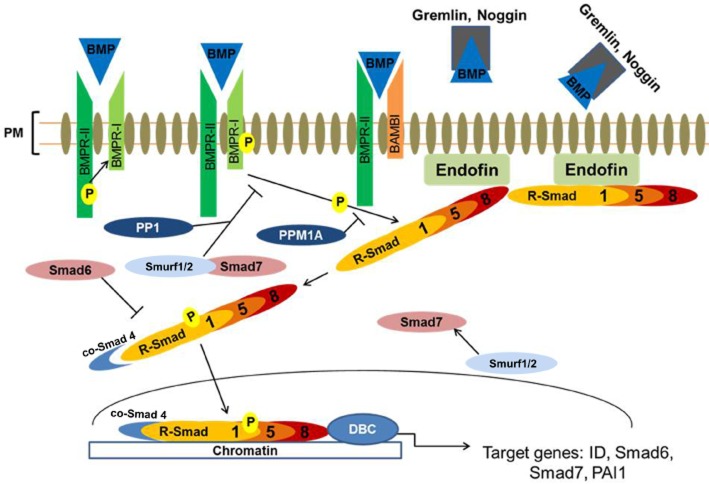
Figure 2.
Activation and regulation of BMP/Smad-dependent signalling. BMP ligands bind to and activate type I and II serine/threonine kinase receptors (BMPR-I and BMPR-II, respectively), which triggers phosphorylation of the R-Smad1/5/8. Phosphorylated R-Smads form a heteromeric complex with the co-Smad 4, enabling its subsequent translocation to the nucleus where it binds to specific GC rich sequences within the promoters of several target genes in concert with various DNA binding co-factors. This cascade is tightly regulated, through pseudo-receptors such as BAMBI, which quench BMP ligands thereby limiting their availability to interact with their receptors. Extracellular regulation occurs via direct interaction of BMPs with their secreted antagonists, thereby preventing ligand receptor interaction. Intracellularly, this pathway is regulated by the I-Smads, Smad6 and 7. Smad6 prevents the formation of R-Smad and co-Smad complex formation. Smad7 regulates this pathway by forming a complex with Smurf1/2 and competing with R-Smad for type I receptor-mediated activation effectively antagonizing BMP/Smad pathway activation. This pathway is also regulated by phosphatases such as PP1, which dephosphorylates the BMP type I receptor, and PPM1A, which dephosphorylates R-Smads. R-Smad, receptor regulated Smads; I-Smad, inhibitory Smads; co-Smad, co-mediator Smad; ID, inhibitor of differentiation; PAI1, plasminogen activator inhibitor-1; PP1, protein phosphatase 1; PPM1A, metal ion-dependent protein phosphatases 1A; DBC, DNA binding co-factors; PM, plasma membrane.