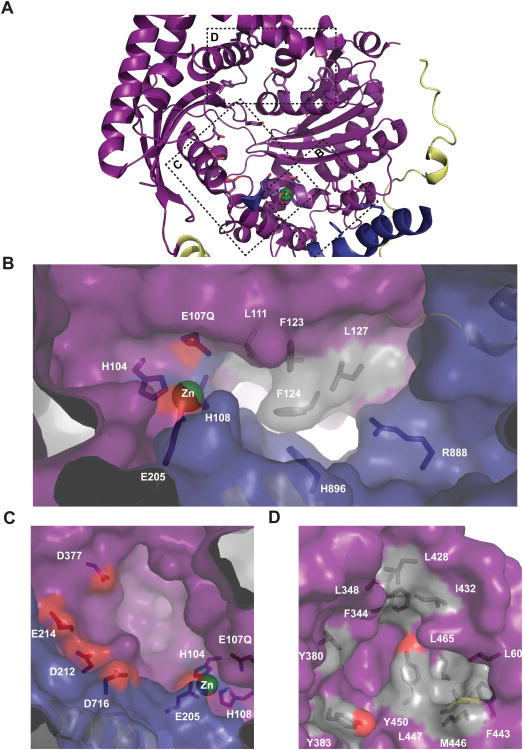
Figure 2. Residues in the hPreP Chamber Facilitate Substrate Recognition.
(A) HPreP-N catalytic chamber. (B) Two pockets (L111, F123, F124, and L127; and H896 and R888) explain observed cleavage site preference for P1 or P′1 hydrophobic residues, and scissile bonds 2-5 residues distal from substrate C-termini. (C) Labeled acidic residues are proximal to the active site and can facilitate interaction with basic substrate residues (D) A network of hydrophobic resides in hPreP-N permits capture of hydrophobic residues.