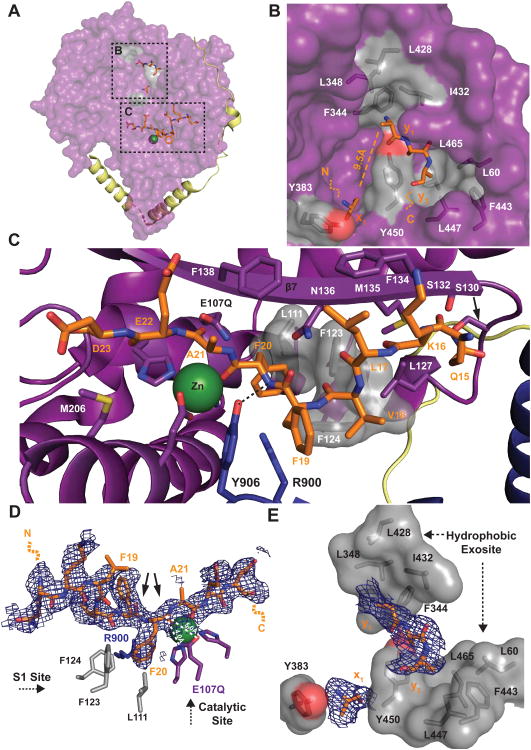
Figure 5. Structural Basis of Aβ Capture and Recognition by HPreP.
(A) Aβ-binding to hPreP-N. (B) Hydrophobic exosite. Four Aβ residues (x1, y1-3) are observed. Hydrophobic residues that comprise the putative side chain binding pockets are displayed in gray. (C) Aβ-binding to the hPreP catalytic cleft. The S1 site consists of L111, F123, F124, and R900. (D-E) 2Fo-Fc electron density maps contoured at 0.5σ for Aβ residues bound to (D) the catalytic cleft and (E) the hydrophobic exosite. Arrows denote preferred Aβ cleavage sites by hPreP. See also Figure S5.