Significance
Hormonally active women are better protected from the primary liver cancer hepatocellular carcinoma (HCC) than men. In rodent models, the pituitary gland governs sexually dimorphic HCC risk. We found that the estrogen-responsive pituitary hormone prolactin (PRL) inhibits HCC by binding liver short-form prolactin receptors, resulting in accelerated degradation of a multimolecular “trafasome” involved in tumor-promoting innate immune signaling and preventing activation of the HCC-associated gene c-Myc. Mouse models confirmed sex-dependent HCC regulation upstream of c-Myc and a requirement for PRL but not estrogen in female tumor resistance. Importantly, a preclinical trial in mice showed that pharmacologic PRL mobilization using the human drug domperidone protected males from HCC. PRL-targeted therapy may help prevent liver cancer in high-risk men and women.
Keywords: liver neoplasms, lactotrophs, innate immunity, sex dimorphism
Abstract
Women are more resistant to hepatocellular carcinoma (HCC) than men despite equal exposure to major risk factors, such as hepatitis B or C virus infection. Female resistance is hormone-dependent, as evidenced by the sharp increase in HCC incidence in postmenopausal women who do not take hormone replacement therapy. In rodent models sex-dimorphic HCC phenotypes are pituitary-dependent, suggesting that sex hormones act via the gonadal-hypophyseal axis. We found that the estrogen-responsive pituitary hormone prolactin (PRL), signaling through hepatocyte-predominant short-form prolactin receptors (PRLR-S), constrained TNF receptor-associated factor (TRAF)-dependent innate immune responses invoked by IL-1β, TNF-α, and LPS/Toll-like receptor 4 (TLR4), but not TRIF-dependent poly(I:C)/TLR3. PRL ubiquitinated and accelerated poststimulatory decay of a “trafasome” comprised of IRAK1, TRAF6, and MAP3K proteins, abrogating downstream activation of c-Myc–interacting pathways, including PI3K/AKT, mTORC1, p38 MAPK, and NF-κB. Consistent with this finding, we documented exaggerated male liver responses to immune stimuli in mice and humans. Tumor promotion through, but regulation above, the level of c-Myc was demonstrated by sex-independent HCC eruption in Alb-Myc transgenic mice. PRL deficiency accelerated liver carcinogenesis in Prl−/− mice of both sexes. Conversely, pharmacologic PRL mobilization using the dopamine D2 receptor antagonist domperidone prevented HCC in tumor-prone C3H/HeN males. Viewed together, our results demonstrate that PRL constrains tumor-promoting liver inflammation by inhibiting MAP3K-dependent activation of c-Myc at the level of the trafasome. PRL-targeted therapy may hold promise for reducing the burden of liver cancer in high-risk men and women.
Liver cancer is the third leading cause of cancer mortality worldwide, and the fastest increasing cancer in the United States (1). Despite equal exposure to major risk factors, such as infection with hepatitis B or C virus, men develop hepatocellular carcinoma (HCC) more than twice as often as women (1). Protection in women is hormone-dependent, as evidenced by the sharp rise in female liver cancer incidence following menopause (2). Hormone replacement therapy (HRT) in postmenopausal women restores HCC resistance (3). Estrogen (17β-estradiol; E2) has been shown to contribute directly to liver tumor inhibition through effects on nonparenchymal hepatic immune cells (4, 5). However, in rodent models the pituitary gland is indispensable to maintain sex-dependent HCC phenotypes (6), suggesting that sex hormones act via the gonadal-hypophyseal axis. Prolactin (PRL) is an E2-responsive pituitary hormone expressed at higher levels in females than males (7). Here, we show that PRL mitigates TNF receptor-associated factor (TRAF)-mediated innate immune responses in hepatocytes, preventing tumor-promoting activation of the liver proto-oncogene c-Myc through multiple MAP3K-dependent pathways, including PI3K/AKT, mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), p38 MAPK, and NF-κB. This mechanism may contribute to HCC resistance in women.
Results
Short-Form Prolactin Receptor Mediates Liver Signaling.
The prolactin receptor (PRLR) has multiple splice variants with tissue-specific expression (8). Long-form PRLR (PRLR-L) is the primary receptor in breast and most other tissues, where it functions in part through STAT5 (9). In contrast, short-form PRLR (PRLR-S) does not meaningfully activate STAT5 or other PRLR-L pathways (10). In female reproductive tissues, the proportion of long:short-form receptor varies depending on hormonal fluctuations, whereas the liver maintains a constant preference for PRLR-S (11–13). In agreement with reports elsewhere (8), we found in mammary tissue and cells that 85–90% of total PRLR mRNA was PRLR-L, whereas in mouse and human liver tissue the vast majority was PRLR-S (Fig. S1A). Murine Hepa 1–6 and AML12 liver cells retained in vivo liver preference for PRLR-S over PRLR-L. In contrast, human liver cell lines, including HCC-derived Hep3B and HuH7, immortalized hepatocyte subclone PH5CH8, and hepatoblastoma-origin HepG2 and HuH6 exhibited variable total PRLR but a universal preference for PRLR-L over PRLR-S (Fig. S1B; all liver cell line origins are depicted in Table S1). Our findings correlated well with a published microarray comparison of PRLR expression in human liver cell lines (14). Based on the above observations, we chose the murine liver cell lines Hepa 1–6 and AML12, except where noted for the functional PRL studies below. There are at least three known splice variants of PRLR-S in mice and two in humans (15, 16). In mice, we found that variant S3 comprised >90% of all PRLR-S (Fig. S1C). In human liver, PRLR-S1a was significantly more abundant than isoform S1b. We confirmed site-specific PRLR isoform expression at the protein level by Western blot (Fig. S1D). To determine the impact of pituitary hormones on STAT5 and MAPK signaling, we withdrew serum from Hepa 1–6 cells and subsequently reintroduced PRL or growth hormone. Phosphorylation of STAT5 and ERK1/2 was not affected by any treatment in these cells (Fig. S1E), whereas serum starvation activated the stress-associated protein kinase p38 MAPK. Intriguingly, PRL but not growth hormone decreased p38 MAPK phosphorylation to levels found in unstressed cells grown with serum. Inhibition of MAP kinases including p38 MAPK was shown elsewhere to be a feature of PRLR-S but not PRLR-L signaling (17). These data highlight PRLR-S as the primary prolactin receptor in differentiated hepatocytes, and show that PRL exposure constrains at least one cell stress-associated pathway.
PRL Inhibits IL-1β Induction of p38 MAPK, NF-κB, and AKT.
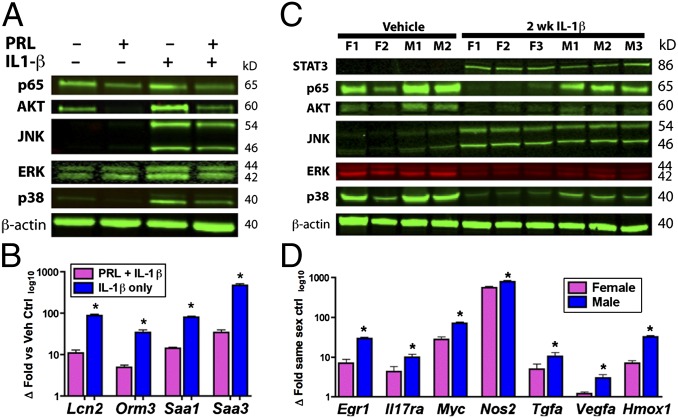
IL-1β is an injury-associated innate immune cytokine implicated in tumor-promoting liver inflammation (18). To begin our search for PRL-sensitive innate immune pathways in hepatocytes, we exposed Hepa 1–6 and AML12 cells with or without PRL preconditioning to recombinant murine IL-1β. In addition to p38 MAPK, we found that PRL exposure decreased IL-1β–induced phosphorylation of AKT and NF-κB p65/RelA, but not ERK1/2 or JNK (Fig. 1A). At the message level, PRL inhibited transcription of important murine acute-phase response (APR) genes in IL-1β–challenged hepatocytes, including serum amyloid A1 and A3, lipocalin-2, and orosomucoid (α-1-acid glycoprotein) (Fig. 1B). The Toll-like receptor (TLR)4 ligand LPS also invoked immune responses in these cells (Fig. S2A). In a time-course study, hepatocyte preconditioning with PRL for 1 h significantly reduced IL-1β– and LPS-induced target phosphorylation, although 2 h was required for maximal inhibition; this effect was durable up to 8 h (Fig. S2B). Because 1–2 h of PRL incubation was required to achieve maximal inhibition, we tested whether PRL interruption of IL-1β responses required de novo transcription. However, by microarray we identified few PRL-inducible genes and no common gene sets shared between Hepa 1–6 and AML12 cells; moreover, we observed no effect by the transcription inhibitor actinomycin D on PRL-sensitive gene expression in either cell line [National Center for Biotechnology Information Gene Expression Omnibus (GEO) SuperSeries accession no. GSE55669]. Viewed together, these data suggest that PRL inhibits IL-1β phosphorylation of p38 MAPK, NF-κB, and AKT in hepatocytes in a transcription-independent manner.
Fig. 1.
Prolactin/female sex constrains hepatocellular IL-1β responses in vitro and in vivo. (A) PRL preconditioning in AML12 hepatocytes reduces IL-1β phosphorylation of p38 MAPK, NF-κB p65/RelA, and AKT. (B) IL-1β induction of APR genes is diminished in PRL-preconditioned hepatocytes. (C) Decreased phosphorylation of p38 MAPK, NF-κB p65/RelA, and AKT but not STAT3, JNK, or ERK1/2 in IL-1β–challenged female versus male mouse liver. (D) Muted induction of inflammatory and tumor-associated genes in IL-1β–challenged females versus males. Data are expressed as mean ± SEM (n = 5 per sex); *P < 0.05, Student t test.
PRL Restricts TLR4 and TNF-α but Not TLR3 Signaling.
To extend our investigation of innate immune pathways susceptible to PRL interruption, we challenged hepatocytes with the TLR3 ligand and dsRNA mimetic poly(I:C), TNF receptor-1 (TNFR1) ligand TNF-α, and LPS using NF-κB p65/RelA phosphorylation as a readout. PRL preconditioning reduced NF-κB activation by LPS and TNF-α but not poly(I:C) (Fig. S2C). In agreement with this, PRL-preconditioned hepatocytes showed muted APR gene expression in the face of LPS challenge, whereas there was no impact by PRL on poly(I:C)-induced APR or TLR3-specific IFN pathway-associated gene transcription (Fig. S2D). Our aggregate findings indicate that PRL dampens hepatocellular immune responses that signal through IL1R, TLR4, and TNFR1, but not TLR3.
Female Mouse Liver Constrains IL-1β Responses.
In humans and in rodent models, females are more resistant to chronic progressive hepatitis and HCC than males (1, 3, 19, 20). To determine whether PRL-sensitive innate immune pathways contribute to this sexual dimorphism, we challenged male and female C3H/HeN mice with IL-1β or poly(I:C). Mirroring results from PRL-preconditioned hepatocytes, females challenged with IL-1β showed reduced phosphorylation of p38 MAPK, NF-κB p65/RelA, and AKT, but not ERK1/2, JNK, or STAT3 compared with males (Fig. 1C). Also reflecting in vitro results, there were no consistent sex-dependent differences in liver gene induction in mice challenged with poly(I:C) (Fig. S2E). We found by microarray that message levels of IL-1β–inducible APR genes in vivo did not exhibit as clear a sex difference as PRL-preconditioned hepatocyte cultures (GEO accession no. GSE55669). This result was likely in part because of sex-independent IL-1β induction of IL-6 (increased >100-fold both sexes) from Küppfer cell macrophages in intact liver; significant redundancy is known to exist between IL-1β– and IL-6–inducible APR pathways (21). APR genes notwithstanding, the impact of IL-1β on transcription of many important inflammatory, cell stress, and growth-associated gene products was muted in female mouse liver, including heme oxygenase-1, early growth response-1, IL-17 receptor-α, inducible nitric oxide synthase (iNOS), TGF-α, VEGF-α, and c-Myc (Fig. 1D). In agreement with a prior study from our group (19), we also observed overrepresentation of sexually dimorphic liver genes among those altered by IL-1β, including feminine genes Cyp2b9, Cyp2b13, Cyp4a10, Cyp4a14, and Sult2a3, as well as masculine genes C6, Cyp4a12, Elovl3, Lcn13, and Spink6 (GEO accession no. GSE55669). Thus, loss of transcriptional regulation of many sex-dependent genes (a process we previously termed “liver-gender disruption”), is an early event in the dichotomous progression of chronic liver disease (19, 22). In summary, in vivo IL-1β challenge results mirrored those observed in vitro, and confirmed that immune induction of tumor-promoting metabolic, stress and cell proliferation pathways exhibits sexual dimorphism at an early stage.
Men Have a Proinflammatory Hepatic Microenvironment.
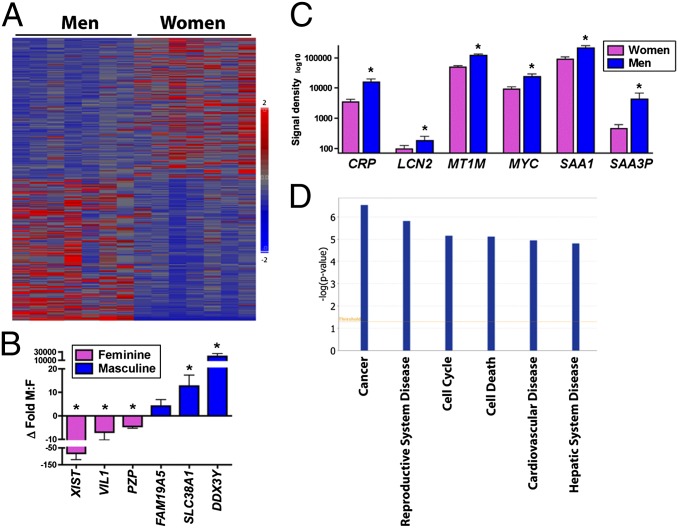
To determine whether sex-dependent proinflammatory liver phenotypes extend to humans, we acquired tissue specimens from seven adult women and men without evidence of primary liver disease (Table S2; donor inclusion criteria in SI Materials and Methods) and performed genome-wide expression analysis (GEO accession no. GSE55669). Heat maps (Fig. 2A) and hierarchal clustering readily differentiated male from female liver. Using additional conservative filtering criteria (SI Materials and Methods), we identified 131 female-predominant and 445 male-predominant unique transcripts (Table S3). We confirmed sex-specificity of selected masculine and feminine liver genes by quantitative RT-PCR (qRT-PCR) (Fig. 2B). Of interest, while analyzing the masculine gene set we noted a large number of canonical APR and inflammation-associated genes as well as several proto-oncogenes, including c-Myc (Fig. 2C). Indeed, of the top six biological function categories distinguishing male from female liver, five were associated with cancer or inflammatory disease; the other was reproduction (Fig. 2D). Whereas our study could not control for lifestyle factors or correlate serum hormone levels, gene expression results suggest that men—even those without clinically evident liver disease—maintain more of a proinflammatory hepatic microenvironment than women. If our animal model data are indicative, then a relative inability of the human male liver to constrain minor immune stimuli may serve as a bellwether of increased sensitivity to tumor-promoting inflammation. This finding agrees with the known higher risk of men with chronic viral hepatitis to progress to cirrhosis or HCC (1, 3).
Fig. 2.
Adult men without primary liver disease maintain a more proinflammatory hepatic microenvironment than women. (A) Heat map demonstrates clear distinction between male and female human liver (n = 7 per sex). (B) qRT-PCR confirmation of sex-specific expression of selected feminine (pink) and masculine (blue) genes. (C) Adult men exhibit increased expression of inflammatory and proto-oncogenes, including c-Myc. (D) Top sex-specific biological function categories are associated with cancer and inflammation. *P < 0.05, Student t test.
PRL Interrupts IL-1β Upstream of p38 MAPK and AKT.
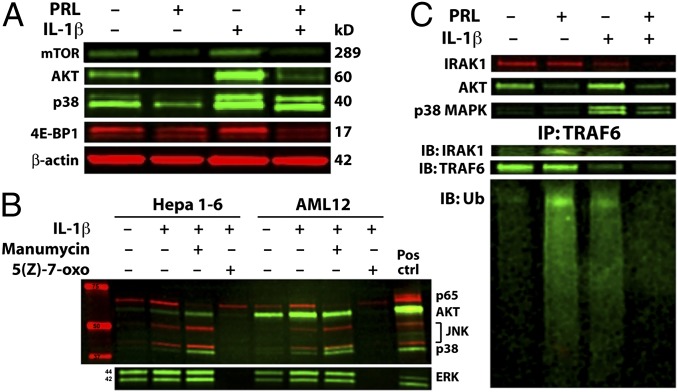
p38 MAPK, NF-κB, and AKT have both overlapping and distinct immune signaling functions. To begin to understand how these pathways cross-talk and how PRL might impact them in the liver, we applied a variety of kinase and phosphatase inhibitors to cultured hepatocytes. However, in our hands the impact of most inhibitors on IL-1β ± PRL signaling was context- and cell line-dependent (Tables S4 and S5). Because the dual-specificity phosphatase (DUSP) DUPD1 previously was implicated in PRLR-S–mediated inhibition of p38 MAPK and ERK1/2 in PRLR-L–deficient mouse reproductive tissues (17), we were particularly interested in the potential role of DUPD1 in hepatocyte PRL signaling. However, none of the kinase or phosphatase inhibitors that target p38 MAPK, including the DUSP inhibitor NSC 95397, gave consistent results between mouse and human cell lines (Table S5). Moreover, neither PRL nor NSC 95397 impacted ERK1/2 phosphorylation, suggesting that a mechanism besides or in addition to DUPD1 mediates PRLR-S activity in hepatocytes. In contrast, the PI3K inhibitors wortmannin and LY294002 consistently abolished AKT phosphorylation, indicating PI3K dependence (Fig. S3A). Using specific inhibitors, we further found that IL-1β activated PI3K/AKT and p38 MAPK pathways independent of one another (Fig. S3B), suggesting that PRL intervenes at a common upstream site. In genetically engineered mice, constitutive liver activation either of PI3K/AKT or its downstream target mTORC1 results in HCC (23). To determine whether PRL interruption of PI3K/AKT also obviates mTORC1, we evaluated IL-1β–inducible phosphorylation of mTORC1 components, including mTOR, glycogen synthase kinase 3 beta (GSK-3β), p70 S6-kinase (S6K), and eukaryotic translation initiation factor 4E binding protein-1 (4E-BP1). Importantly, PRL interruption of PI3K/AKT also abrogated phosphorylation of mTOR and 4E-BP1 (Fig. 3A). We were unable to detect phospho-specific GSK-3β or S6K under any of our experimental conditions. Taken together, our results demonstrate that PRL interrupts IL-1β signaling at a node upstream of p38 MAPK and PI3K, and reveal that constraint of PI3K/AKT also alleviates downstream mTORC1.
Fig. 3.
PRL inhibits immune induction of MAP3K-dependent pathways including PI3K/AKT/mTORC1 by accelerating degradation of trafasome. (A) PRL constrains mTORC1 members mTOR and 4E-BP1 in parallel with AKT. (B) MAP3K inhibitor 5(Z)-7-oxozeaenol, but not Ras inhibitor manumycin, mirrors PRL in disruption of p38 MAPK, AKT, and NF-κB p65/RelA phosphorylation. (C, Upper) Western blot demonstrates that prior PRL accelerates degradation of IRAK1 following IL-1β challenge in AML12 cells. (Lower) TRAF6 immunoprecipitation (IP) followed by immunoblot (IB) shows nondegrading ubiquitination by PRL alone, and accelerated degradation of IRAK1 and TRAF6 following IL-1β challenge.
PRL Targets Are MAP3K- but Not Ras-Dependent.
Ras is a well-characterized oncogene that acts in part through PI3K/AKT, whereas MAP3K enzymes (notably TAK1) may simultaneously target NF-κB and p38 MAPK (24). To clarify the role of these pathways in our system, we used the Ras inhibitor manumycin and MAP3K inhibitor 5(Z)-7-oxozeaenol. Unexpectedly, we discovered that Ras inhibition increased phosphorylation of all IL-1β targets except NF-κB (Fig. 3B). In contrast, the MAP3K inhibitor abolished endogenous and IL-1β–inducible phosphorylation of all targets (except some residual Ras-dependent NF-κB). At nanomolar concentrations, 5(Z)-7-oxozeaenol is a TAK1-specific antagonist; however, at the 10-μM concentration we used it inhibits other MAP3K (25). We found that decreasing the concentration of 5(Z)-7-oxozeaenol below 10 μM diminished inhibition of IL-1β target activation in a dose-dependent fashion (Fig. S3C). Therefore, one or more MAP3K besides or in addition to TAK1 must transmit PRL-sensitive IL-1β signals in hepatocytes. Further work will be required to identify which of the >20 MAP3K participate in PRL-sensitive hepatocellular signaling (26). Nevertheless, our data show that innate immune pathways interrupted by PRL are MAP3K-dependent.
PRL Primes “Trafasome” for Poststimulatory Degradation.
Based on the results above, we directed our search for molecular PRL targets toward MAP3K-dependent pathways shared by IL1R, TLR4, and TNFR1, but not TLR3. Of note, all of the PRL-sensitive pathways listed above, but not PRL-insensitive TLR3, involve TRAF proteins. This finding is significant because TRAF proteins anchor modular signaling complexes (“trafasomes”) (27) that may incorporate any of several MAP3K (28, 29). Trafasomes rely heavily on context-specific ubiquitination for activation and signal termination (30). To determine the impact of PRL on trafasome kinetics, we immunoprecipitated TRAF6 from hepatocytes exposed to PRL or IL-1β. As expected, IL-1β recruited interleukin-1 receptor-associated kinase 1 (IRAK1) to the trafasome, resulting in TRAF6 ubiquitination and decreased total protein consistent with proteasomal degradation or autophagy. Unexpectedly, we found that PRL alone also recruited IRAK1 to TRAF6, invoking nondegrading ubiquitination without appreciable loss of either protein (Fig. 3C). Of greatest significance, we found in PRL-preconditioned hepatocytes that virtually no IRAK1 or TRAF6 could be detected 15 min after IL-1β stimulation, indicating accelerated decay. Our combined results outline a model whereby PRL recruits IRAK1 to TRAF, resulting in sensitizing ubiquitination and accelerated degradation of the trafasome following immune stimulation; this in turn terminates downstream MAP3K-dependent signaling through p38 MAPK, NF-κB, and PI3K/AKT/mTORC1.
In Vivo Sex-Dependent HCC Is Regulated Above c-Myc.
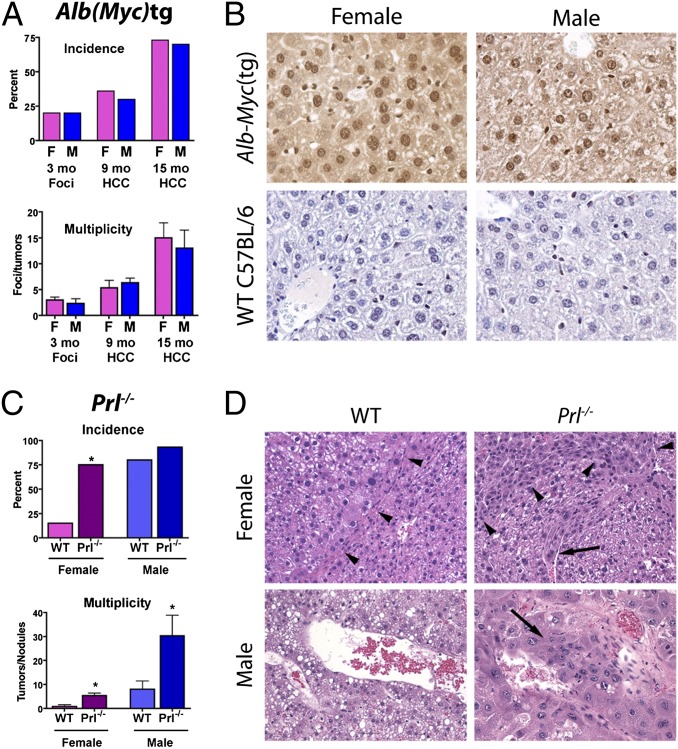
In addition to immunity, p38 MAPK, NF-κB, and PI3K/AKT/mTORC1 have known associations with cancer in part through induction of c-Myc (31). Of importance, c-Myc is up-regulated in male-specific fashion at an early stage in rodent hepatocarcinogenesis (32). If the present hypothesis is correct and PRL impedes liver tumorigenesis at the level of the trafasome, then release of c-Myc from immunoregulatory control should abolish sex-dependent HCC phenotypes. To test this theory, we monitored spontaneous tumor development in mice with constitutive hepatic c-Myc expression under control of the sex-independent albumin promoter/enhancer (33). To obviate any potential confounding by proinflammatory gut microbes, mice were housed in a Helicobacter-free barrier facility (22). Under these conditions, we found no sex difference in the incidence or multiplicity of preneoplastic foci of cellular alteration (FCA) at 3 mo or frank neoplasms at 9 and 15 mo (Fig. 4A). Functional transgene expression was confirmed by immunohistochemistry in 3-mo-old male and female mice (Fig. 4B). Results of this experiment are consistent with universal liver tumor promotion through—but sex-dependent regulation above—the level of c-Myc.
Fig. 4.
Female HCC resistance is PRL-dependent and regulated above c-Myc. (A) Absence of sex bias in preneoplastic foci and HCC in Alb-Myc(tg) mice with constitutive hepatic c-Myc expression. (B) Immunohistochemical confirmation of cytoplasmic and nuclear c-Myc overexpression (diaminobenzidine; brown) in transgenic mice of both sexes compared with WT controls (magnification, 400×). (C) Increased HCC incidence (Upper) and multiplicity (Lower) in PRL-deficient mice relative to WT controls. (D) DEN-initiated Prl−/− mice have greater local (arrowheads) and vascular (arrows) tumor invasion than sex-matched WT controls (magnification, 200×). Data are expressed as mean ± SEM (n = 9–10 per sex per treatment protocol), *P < 0.05, one-way ANOVA with Tukey posttest.
PRL Deficiency Accelerates Liver Tumorigenesis.
If PRL constrains liver tumorigenesis, then PRL deficiency should increase HCC vulnerability. To test this hypothesis, we inoculated infant C57BL/6 and strain-matched Prl−/− mice with the liver carcinogen diethylnitrosamine (DEN) or vehicle. Compared with WT controls, DEN-initiated Prl−/− females developed a significantly higher incidence and multiplicity of liver tumors (Fig. 4C). Moreover, neoplasms from Prl−/− females exhibited increased malignancy, as indicated by invasion of adjacent nonneoplastic tissue and vessels (Fig. 4D). Because factors in addition to PRL contribute to male HCC vulnerability (i.e., disturbances arising from growth hormone-mediated liver masculinization) (19), Prl−/− males still developed a greater tumor burden than females. Nevertheless, PRL deficiency also increased the multiplicity and malignancy of HCC in Prl−/− males. Importantly, it has been shown that Prl−/− female mice maintain circulating E2, albeit at lower levels than WT mice (34), indicating a requirement for PRL in hormone-based tumor inhibition. Viewed together, results from Prl−/− mice indicate that PRL deficiency accelerates hepatocarcinogenesis in both sexes, but is especially pronounced in females.
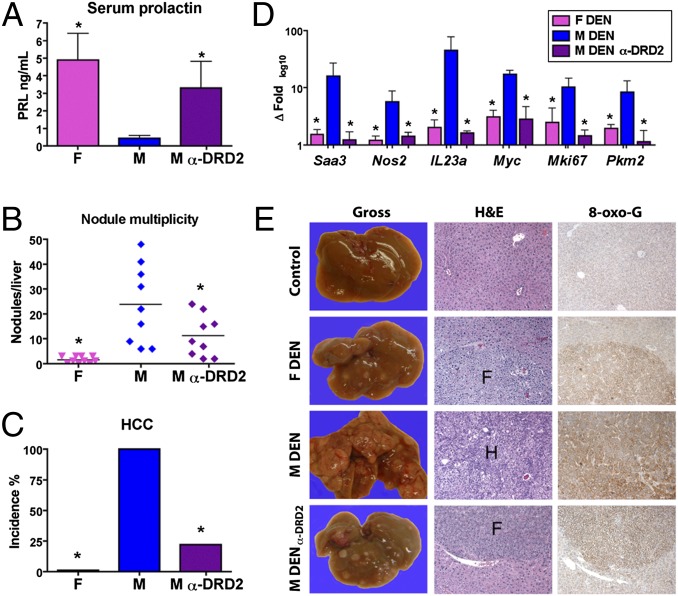
Pharmacologic PRL Mobilization Protects Males from HCC.
Estrogen-based HRT protects postmenopausal women from liver cancer (3), likely in part through PRL induction. However, HRT is not a viable strategy for liver cancer prevention in men. To prove in principle that E2-independent PRL agonism can protect males from HCC, we initiated tumor-prone C3H/HeN mice with DEN or vehicle, and administered the PRL-mobilizing dopamine D2 receptor antagonist (α-DRD2) domperidone to a cohort of males. Domperidone was withheld until mice had developed preneoplastic FCA to recapitulate tumor risk profiles characteristic of humans with chronic progressive hepatitis or cirrhosis. As expected, domperidone increased serum PRL in males to near-female levels (Fig. 5A). All DEN-initiated untreated males developed HCC, whereas females presented with FCA and dysplastic nodules only. The multiplicity of grossly evident dysplastic nodules in domperidone-treated mice was intermediate between females and untreated males (Fig. 5B). However, with regard to HCC, DEN-initiated males that received α-DRD2 therapy showed significant protection (22% incidence vs. 100% in untreated males; P < 0.01) (Fig. 5C). Moreover, message levels of inflammation-associated gene products, including SAA3, iNOS, and IL-23α were decreased in domperidone-treated males to feminine levels, along with tumor-associated biomarkers c-Myc, Ki-67, and pyruvate kinase M2 (Fig. 5D) (35). Lesions from domperidone-treated males also more closely resembled female preneoplastic foci with regard to morphology and oxidative stress biomarkers (e.g., 8-oxoguanine) than the frank malignancies from untreated males (Fig. 5E). Combined with studies elsewhere involving the PRL-mobilizing agents perphenazine and cimetidine (36, 37), our results establish E2-independent PRL induction as a rational strategy to prevent HCC.
Fig. 5.
PRL-mobilizing DRD2 antagonist domperidone prevents male HCC. (A) α-DRD2 domperidone increases circulating male PRL to near female levels. (B) Domperidone in male mice reduces multiplicity of dysplastic nodules to intermediate levels between untreated males and females. (C) Domperidone markedly reduces male HCC incidence (22% vs. 100% in untreated males). (D) Tumor-associated transcriptional and morphologic biomarkers in α-DRD2–treated males mirror those of females. (E) Gross and histologic appearance of DEN-induced lesions in α-DRD2 males approximate those found in females. F, foci of cellular alteration; H, HCC; 8-oxo-G, 8-oxoguanine; magnification, 100×. Data are represented as mean ± SEM, *P < 0.05 (n = 9–10 mice per sex per treatment protocol), one-way ANOVA with Tukey posttest.
Discussion
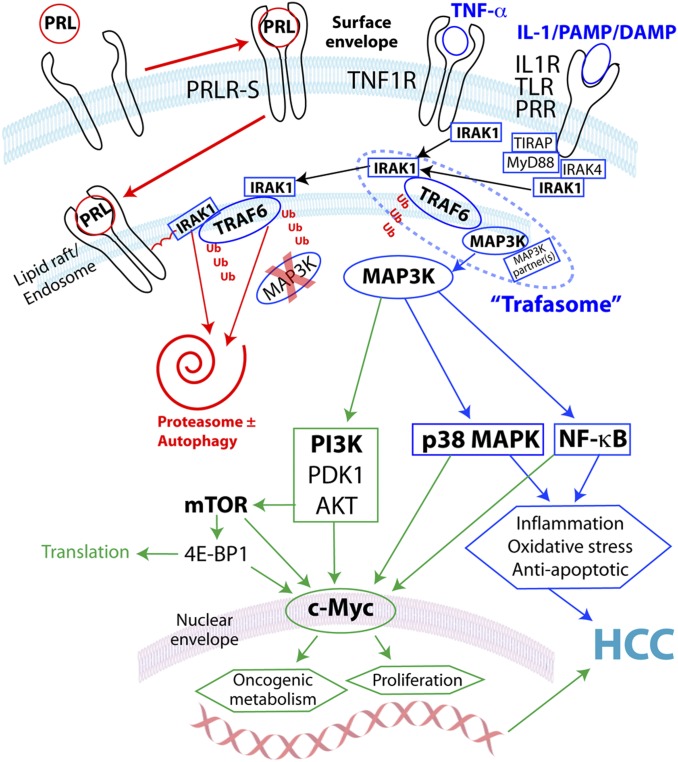
Hormonally active women are better protected from HCC than men and postmenopausal women who do not take HRT (3). Our study implicates a crucial role for prolactin in female HCC resistance. PRL interrupted innate immune pathways in hepatocytes mediated by TRAF-dependent IL-1β, LPS/TLR4, and TNF-α, but not TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent poly(I:C)/TLR3. We found that PRL alone invoked sensitizing ubiquitination of a trafasome comprised of IRAK1, TRAF6, and MAP3K proteins, leading to accelerated complex degradation following immune stimulation. This process prevented activation of c-Myc via multiple MAP3K-dependent pathways, including p38 MAPK, NF-κB, and PI3K/AKT/mTORC1 (Fig. 6). We hypothesize that PRL function in liver is mediated predominantly by PRLR-S. Our finding of weak transcriptional induction by PRL in murine hepatocytes is consistent with the limited impact of PRL on STAT5 signaling in mouse liver (38). In vivo, circadian/ultradian hormone secretory patterns regulate gene transcription kinetics (39, 40). It is possible that ultradian secretion of PRL (7) also influences extranuclear functions of PRLR-S. Delayed onset but persistent activity of PRL in our model (Fig. S2B) is consistent with the slow subcellular trafficking and diminuted turnover of ligand-bound PRLR-S compared with PRLR-L (41). Additional work will be required to parse the role of different hormones and receptors in the complex process of human liver carcinogenesis. Nevertheless, our model reconciles previously disconnected rodent studies, and introduces a mechanism by which an E2-responsive pituitary hormone may help prevent HCC.
Fig. 6.
Working model of PRL interruption of TRAF/MAP3K-dependent innate immune activation of the proto-oncogene c-Myc via PI3K/AKT/mTORC1, p38 MAPK and/or NF-κB in hepatocytes. Inhibitory pathways shown in red, inflammatory in blue and proliferative in green. Abbreviations: DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern; PRR, pattern-recognition receptor.
Our results confirm and extend reports from other groups showing reduced HCC in mice and rats with PRL mobilization via pituitary allograft or pharmacologic induction (36, 37). However, another study reported no impact of PRL receptor deficiency on DEN-initiated liver carcinogenesis in Prlr−/− mice (42). There are several potential explanations for this apparent discrepancy. First, Prl−/− and Prlr−/− mice show numerous biological differences, including energy metabolism, reproductive viability, and behavior (43–45). Therefore, PRL ligand and receptor knockout mice have different phenotypes. Second, Prlr−/− mice acquire extremely high levels of circulating PRL because of an absence of negative feedback inhibition (46), which could result in off-target effects. Finally, the Prlr−/− study limited analysis to grossly visible liver masses, whereas histopathology allowed us to distinguish preneoplastic nodules from bona fide malignancies, which proved to be an important differentiator (Fig. 4D). Our findings also contrast with a paper that correlated JAK2 with human HCC, and implied a role for PRL signaling in tumor progression (47). However, the association between serum PRL and HCC in patients was correlative only and did not take into account circulating E2. Additionally, the in vitro experiments relied on HepG2 cells, a cell line derived from hepatoblastoma and not HCC (48), and which preferentially express PRLR-L over PRLR-S (Fig. S1B). Finally, the only STAT sensitive to chemical (AG490) or siRNA JAK2 knockdown in their system was STAT3, not PRLR-associated STAT5. Because JAK2 is an upstream kinase for STAT3 as well as STAT5 (49), it is possible that the role of JAK2 in human HCC involves non-PRLR pathways. Further work will be needed to reconcile our findings with both studies above.
In summary, we have shown that PRL impedes liver carcinogenesis by restricting tumor-promoting innate immune responses within hepatocytes. Important questions remain, including whether hepatic PRL signaling is strictly PRLR-S–dependent, how ligand-bound PRLR recruits IRAK1 to the trafasome, whether TRAF molecules other than TRAF6 are targeted, and which MAP3K are involved in PRL-sensitive injury responses in hepatocytes. Furthermore, it is important to note that our model is not incompatible with studies showing E2-mediated HCC inhibition through alternative mechanisms (4, 5). Nevertheless, our preclinical data show that E2-independent PRL mobilization, using a generic drug already in human use, is sufficient to protect males from liver cancer. PRL-targeted therapy may help prevent HCC in high-risk men and women.
Materials and Methods
C3H/HeN mice were purchased from the National Cancer Institute Frederick National Laboratory Production Program (Frederick, MD) and C57BL/6 mice from the Jackson Laboratory. Generation of Albumin-Myc–transgenic [Alb-Myc(tg)] and Prl−/− mice has been previously described (33, 45). Liver collections, tumor evaluation, histopathology, and molecular evaluations were performed as described in SI Materials and Methods and according to previously published techniques (50). Protein and nucleic acid isolation, immunoprecipitation, Western blot, and PCR were performed according to the manufacturers’ protocols. Cell lines except where indicated were obtained from the University of North Carolina at Chapel Hill (UNC) Lineberger Tissue Culture Facility and maintained according to ATCC guidelines. Deidentified human liver biopsies according a protocol approved by the UNC Institutional Review Board were obtained through the UNC Tissue Procurement Facility. In vivo sample sizes were projected to yield a study power ≥0.9 using StatMate software (GraphPad). Statistical evaluations were performed by one-way ANOVA with Tukey posttest, or Student t test using Prism software (GraphPad). Animal euthanasia was performed in accordance with the American Veterinary Medical Association Panel on Euthanasia, and protocols were approved by the Institutional Animal Care and Use Committees of UNC and the Massachusetts Institute of Technology. Complete details are presented in SI Materials and Methods. See Table S6 for all qRT-PCR primer sequences.
Supplementary Material
Acknowledgments
We thank S. Thorgeirsson (National Cancer Institute) for Albumin-Myc–transgenic mice, and S. Earp (University of North Carolina at Chapel Hill) for mammary epithelial cell lines. This work was supported by North Carolina Translational and Clinical Sciences Institute Grant 10KR10818 (to A.B.R.) and National Institutes of Health Grants CA158661 (to A.B.R.), CA016086 sub 8715 (to A.B.R.), CA067529 (to J.G.F.), OD011141 (to J.G.F.), and DK052134 (to N.D.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE55669).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404267111/-/DCSupplemental.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273, e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mucci LA, et al. Age at menarche and age at menopause in relation to hepatocellular carcinoma in women. BJOG. 2001;108(3):291–294. doi: 10.1111/j.1471-0528.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 3.Yu MW, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38(6):1393–1400. doi: 10.1016/j.hep.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, et al. Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs) J Biol Chem. 2012;287(48):40140–40149. doi: 10.1074/jbc.M112.348763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porsch Hällström I, Svensson D, Blanck A. Sex-differentiated deoxycholic acid promotion of rat liver carcinogenesis is under pituitary control. Carcinogenesis. 1991;12(11):2035–2040. doi: 10.1093/carcin/12.11.2035. [DOI] [PubMed] [Google Scholar]
- 7.Vekemans M, Robyn C. Influence of age on serum prolactin levels in women and men. BMJ. 1975;4(5999):738–739. doi: 10.1136/bmj.4.5999.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagano M, Kelly PA. Tissue distribution and regulation of rat prolactin receptor gene expression. Quantitative analysis by polymerase chain reaction. J Biol Chem. 1994;269(18):13337–13345. [PubMed] [Google Scholar]
- 9.Britschgi A, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: A rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22(6):796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Jahn GA, et al. In vivo study of prolactin (PRL) intracellular signalling during lactogenesis in the rat: JAK/STAT pathway is activated by PRL in the mammary gland but not in the liver. Biol Reprod. 1997;57(4):894–900. doi: 10.1095/biolreprod57.4.894. [DOI] [PubMed] [Google Scholar]
- 11.Le JA, et al. Generation of mice expressing only the long form of the prolactin receptor reveals that both isoforms of the receptor are required for normal ovarian function. Biol Reprod. 2012;86(3):86. doi: 10.1095/biolreprod.111.095927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Telleria CM, et al. The different forms of the prolactin receptor in the rat corpus luteum: Developmental expression and hormonal regulation in pregnancy. Endocrinology. 1997;138(11):4812–4820. doi: 10.1210/endo.138.11.5479. [DOI] [PubMed] [Google Scholar]
- 13.Bridges RS, Scanlan VF, Lee JO, Byrnes EM. Reproductive experience alters prolactin receptor expression in mammary and hepatic tissues in female rats. Biol Reprod. 2011;85(2):340–346. doi: 10.1095/biolreprod.111.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staib F, et al. CellMinerHCC: A microarray-based expression database for hepatocellular carcinoma cell lines. Liver Int. 2014;34(4):621–631. doi: 10.1111/liv.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraris J, et al. Prolactin receptor antagonism in mouse anterior pituitary: Effects on cell turnover and prolactin receptor expression. Am J Physiol Endocrinol Metab. 2012;302(3):E356–E364. doi: 10.1152/ajpendo.00333.2011. [DOI] [PubMed] [Google Scholar]
- 16.Qazi AM, Tsai-Morris CH, Dufau ML. Ligand-independent homo- and heterodimerization of human prolactin receptor variants: Inhibitory action of the short forms by heterodimerization. Mol Endocrinol. 2006;20(8):1912–1923. doi: 10.1210/me.2005-0291. [DOI] [PubMed] [Google Scholar]
- 17.Devi YS, et al. Inhibition of MAPK by prolactin signaling through the short form of its receptor in the ovary and decidua: Involvement of a novel phosphatase. J Biol Chem. 2011;286(9):7609–7618. doi: 10.1074/jbc.M110.166603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negash AA, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9(4):e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers AB, et al. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 2007;67(24):11536–11546. doi: 10.1158/0008-5472.CAN-07-1479. [DOI] [PubMed] [Google Scholar]
- 20.Theve EJ, et al. Sex hormone influence on hepatitis in young male A/JCr mice infected with Helicobacter hepaticus. Infect Immun. 2008;76(9):4071–4078. doi: 10.1128/IAI.00401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinton LJ, et al. Hepatocyte-specific mutation of both NF-κB RelA and STAT3 abrogates the acute phase response in mice. J Clin Invest. 2012;122(5):1758–1763. doi: 10.1172/JCI59408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox JG, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2010;59(1):88–97. doi: 10.1136/gut.2009.183749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon S, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5(217):ra24. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, et al. Iron causes interactions of TAK1, p21ras, and phosphatidylinositol 3-kinase in caveolae to activate IkappaB kinase in hepatic macrophages. J Biol Chem. 2007;282(8):5582–5588. doi: 10.1074/jbc.M609273200. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya-Tsuji J, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278(20):18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 26.Craig EA, Stevens MV, Vaillancourt RR, Camenisch TD. MAP3Ks as central regulators of cell fate during development. Dev Dyn. 2008;237(11):3102–3114. doi: 10.1002/dvdy.21750. [DOI] [PubMed] [Google Scholar]
- 27.Schneider M, et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat Immunol. 2012;13(9):823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki K, et al. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal. 2009;2(93):ra66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- 29.Korchnak AC, Zhan Y, Aguilar MT, Chadee DN. Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell Signal. 2009;21(11):1620–1625. doi: 10.1016/j.cellsig.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pineda G, Ea CK, Chen ZJ. Ubiquitination and TRAF signaling. Adv Exp Med Biol. 2007;597:80–92. doi: 10.1007/978-0-387-70630-6_7. [DOI] [PubMed] [Google Scholar]
- 31.Colak D, et al. Integrative and comparative genomics analysis of early hepatocellular carcinoma differentiated from liver regeneration in young and old. Mol Cancer. 2010;9:146. doi: 10.1186/1476-4598-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo M, et al. Two-stage model of chemically induced hepatocellular carcinoma in mouse. Oncol Res. 2013;20(11):517–528. doi: 10.3727/096504013X13747716581336. [DOI] [PubMed] [Google Scholar]
- 33.Sandgren EP, Quaife CJ, Pinkert CA, Palmiter RD, Brinster RL. Oncogene-induced liver neoplasia in transgenic mice. Oncogene. 1989;4(6):715–724. [PubMed] [Google Scholar]
- 34.Cruz-Soto ME, Scheiber MD, Gregerson KA, Boivin GP, Horseman ND. Pituitary tumorigenesis in prolactin gene-disrupted mice. Endocrinology. 2002;143(11):4429–4436. doi: 10.1210/en.2002-220173. [DOI] [PubMed] [Google Scholar]
- 35.Hines IN, et al. Insulin resistance and metabolic hepatocarcinogenesis with parent-of-origin effects in A×B mice. Am J Pathol. 2011;179(6):2855–2865. doi: 10.1016/j.ajpath.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto R, et al. Correlation between serum prolactin levels and hepatocellular tumorigenesis induced by 3′-methyl-4-dimethylaminoazobenzene in mice. Br J Cancer. 1995;72(1):17–21. doi: 10.1038/bjc.1995.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furuta K, et al. Anti-tumor effects of cimetidine on hepatocellular carcinomas in diethylnitrosamine-treated rats. Oncol Rep. 2008;19(2):361–368. [PubMed] [Google Scholar]
- 38.Choi HK, Waxman DJ. Growth hormone, but not prolactin, maintains, low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology. 1999;140(11):5126–5135. doi: 10.1210/endo.140.11.7106. [DOI] [PubMed] [Google Scholar]
- 39.Stavreva DA, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11(9):1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugathan A, Waxman DJ. Genome-wide analysis of chromatin states reveals distinct mechanisms of sex-dependent gene regulation in male and female mouse liver. Mol Cell Biol. 2013;33(18):3594–3610. doi: 10.1128/MCB.00280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu JC, Scott P, Strous GJ, Schuler LA. Multiple internalization motifs differentially used by prolactin receptor isoforms mediate similar endocytic pathways. Mol Endocrinol. 2002;16(11):2515–2527. doi: 10.1210/me.2002-0077. [DOI] [PubMed] [Google Scholar]
- 42.Bigsby RM, Caperell-Grant A. The role for estrogen receptor-α and prolactin receptor in sex-dependent DEN-induced liver tumorigenesis. Carcinogenesis. 2011;32(8):1162–1166. doi: 10.1093/carcin/bgr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaPensee CR, et al. The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology. 2006;147(10):4638–4645. doi: 10.1210/en.2006-0487. [DOI] [PubMed] [Google Scholar]
- 44.Carré N, Binart N. Prolactin and adipose tissue. Biochimie. 2014;97:16–21. doi: 10.1016/j.biochi.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Horseman ND, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16(23):6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuff KG, et al. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanisms. J Clin Invest. 2002;110(7):973–981. doi: 10.1172/JCI15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh YT, Lee KT, Tsai CJ, Chen YJ, Wang SN. Prolactin promotes hepatocellular carcinoma through Janus kinase 2. World J Surg. 2012;36(5):1128–1135. doi: 10.1007/s00268-012-1505-4. [DOI] [PubMed] [Google Scholar]
- 48.Tao J, et al. Activation of β-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JS, et al. JAK2-STAT3 blockade by AG490 suppresses autoimmune arthritis in mice via reciprocal regulation of regulatory T Cells and Th17 cells. J Immunol. 2014;192(9):4417–4424. doi: 10.4049/jimmunol.1300514. [DOI] [PubMed] [Google Scholar]
- 50.Rogers AB, Houghton J. Helicobacter-based mouse models of digestive system carcinogenesis. Methods Mol Biol. 2009;511:267–295. doi: 10.1007/978-1-59745-447-6_11. [DOI] [PubMed] [Google Scholar]