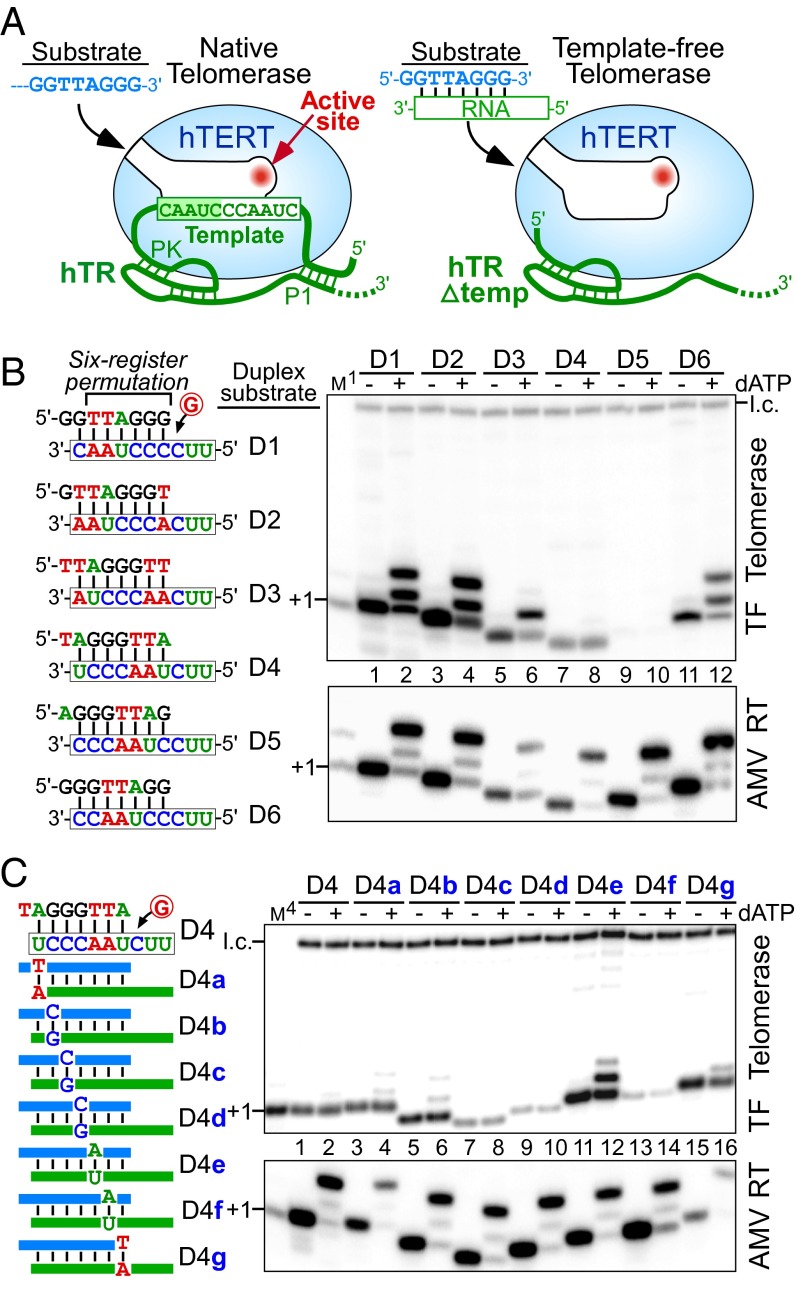
Fig. 1.
A single nucleotide in the RNA/DNA duplex signals a pause in nucleotide addition with template-free (TF) telomerase. (A) Schematic comparison of native (Left) and TF (Δtemp, Right) human telomerases. In the native telomerase, the RNA template is tethered to the 5′ P1 helix and the 3′ pseudoknot (PK) structures. TF telomerase was reconstituted in vitro with hTERT and a 5′ truncated hTR (Δtemp) that lacks the template and the P1 helix. Substrates for the activity assay are single-stranded DNA for native telomerase or a preannealed RNA/DNA duplex for TF telomerase. (B and C, Left) Sequences of permuted telomeric duplexes D1–D6 or D4 substitution variants. (B and C, Right) Activity assay of in vitro reconstituted TF telomerase (Upper) and AMV RT (Lower) with various duplex substrates. Substrates were extended by the enzyme with [α-32P]dGTP in the presence (+) or absence (−) of 0.5 mM dATP as denoted above the gel. A 32P end-labeled 18-mer oligonucleotide was included as a loading control (l.c.). The DNA primers GGTTAGGG (M1) or TAGGGTTA (M4) extended by one [α-32P]dGTP with terminal deoxynucleotidyl transferase (TdT) were included as size markers.