Fig. 3.
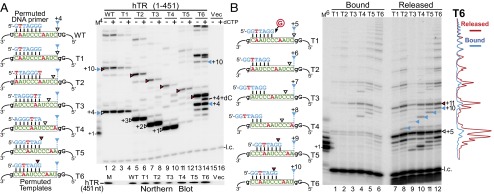
In vivo reconstituted telomerases with permutated templates exhibit sequence-defined nucleotide addition pausing. (A and B, Left) Sequences of the DNA primer and the hTR template variants for telomerase direct assay. The sequence-defined pausing sites before (white triangles) or after (red triangles) template translocation are denoted. The pausing sites at the P1-defined physical template boundary are also denoted (blue triangles). (A, Right) Activity assay for telomerase with template permutations. Telomerases reconstituted in 293FT cells with full-length hTR containing permuted template sequences were assayed with similarly permuted DNA primers in the presence (+) or absence (−) of 0.5 mM dCTP. The DNA primer AGGGTTA extended by one [α-32P]dGTP with TdT was included as a size marker (M4). The vector-only transfected cells (Vec, lanes 15 and 16) were included as a negative control. Northern blot for hTR coimmunopurified with FLAG-tagged TERT is shown under the activity gel. T7-transcribed hTR (451 nt) was included as a size marker (M). (B, Right) Analysis of bound and released DNA products. In vivo reconstituted telomerases (T1–T6) were immobilized on anti-FLAG beads and assayed with a 7-mer DNA primer. DNA products released were separated from those bound to the immobilized enzyme and analyzed by gel electrophoresis. A 6-mer 32P end-labeled oligonucleotide was added to the reaction mix before separation from the beads and a secondary 7-mer 32P end-labeled oligonucleotide was added during phenol/chloroform extraction. Intensity traces of released (red) and bound (blue) products from the T6 template mutant are shown to the right of the gel. The DNA primer GGTTAGG extended by one [α-32P]dGTP with TdT was included as a size marker (M6).
