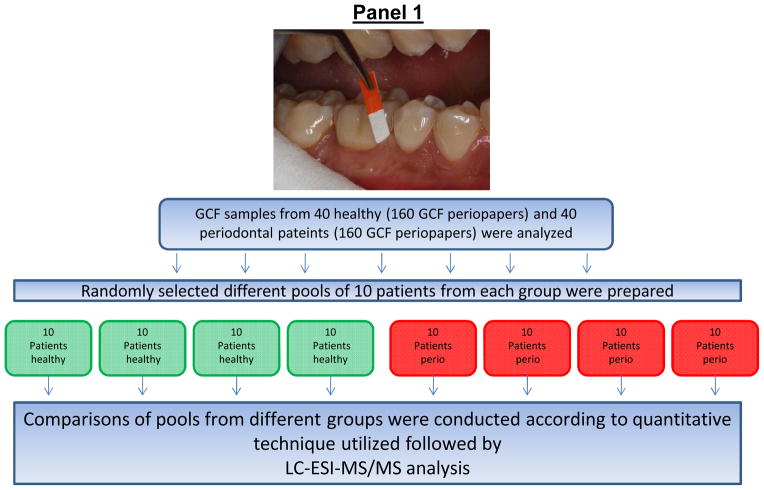
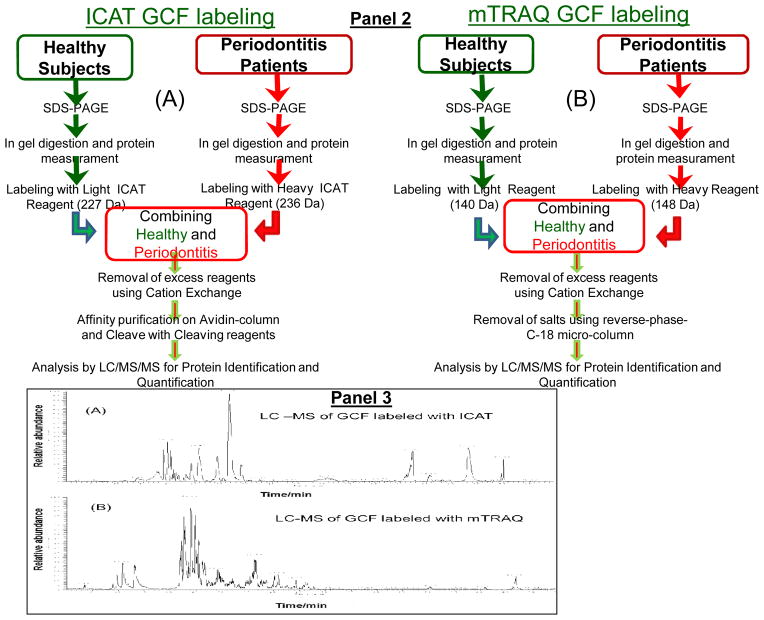
Figure 1. Schematic representation of sample pooling and stable-isotope labeling of GCF samples from healthy and periodontal subjects with ICAT or mTRAQ reagents and processing for LC-ESI-MS/MS analysis.
Panel 1: Different pools of GCF from 10 random patients from each group were prepared for stable-isotope-labeling followed by relative quantitative proteome analysis using LC-ESI-MS/MS. Panel 2: Sequential steps used for stable-isotope labeling of GCF samples for LC-ESI-MS/MS analysis, (A), ICAT and (B), mTRAQ. Panel 3: LC-ESI-MS/MS base-peak ion chromatogram of GCF samples labeled with ICAT and mTRAQ. (A), GCF samples labeled with ICAT, note the simplicity of the LC-MS/MS of ICAT labeled GCF sample illustrating the reduced number of peptides observed since not all of the peptides contain cysteine residues and those peptides without cysteine residues were eliminated during affinity enrichment, and (B), GCF samples labeled with mTRAQ where all of the trypsin peptides are labeled and present.