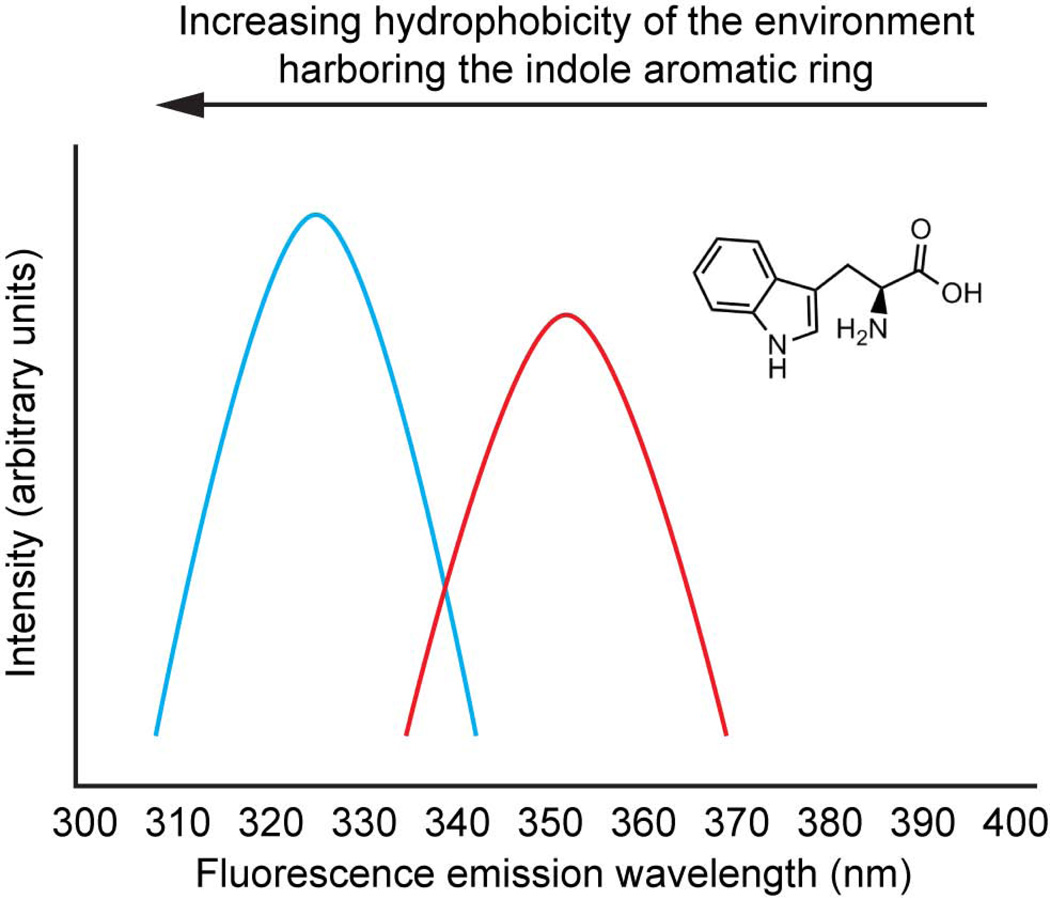
Figure 1.
The indole aromatic side chain of tryptophan is a good example of an environmentally-sensitive fluorophore, one that exhibits a difference between the dipole moment of the ground and electronic excited states. Thus, its emission maximum wavelength is environment sensitive. When placed in the hydrophobic environment of a folded hypothetical protein, the indole ring often emits around 330 nm (although the emission can be blue shifted to as far as 308 nm) owing to a relatively destabilized excited state free energy. Upon chaotrope-mediated hypothetical protein denaturation, a red-shifted indole ring fluorescence emission spectrum is generally observed, owing to the ability of water to lower the excited state free energy, relative to the higher electronic excited state free energy of the indole placed in the hydrophobic core of a protein.