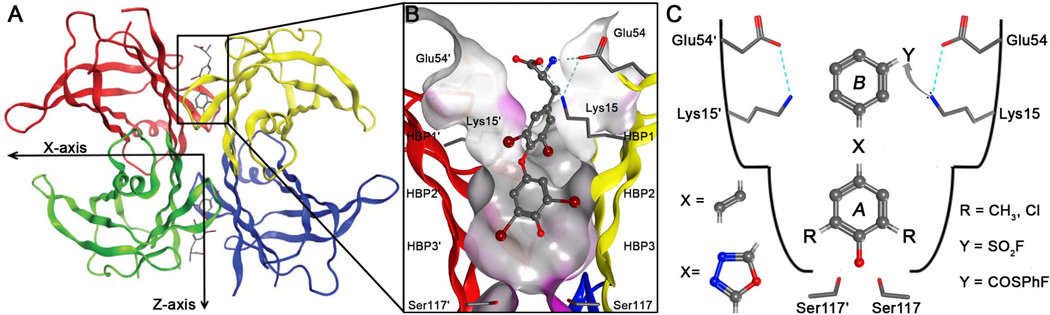
Figure 4.
Structure of homotetrameric WT-TTR with a focus on the T4 binding pocket and pKa-perturbed Lys15 and 15′ residues. (A) Crystal structure of WT-TTR in complex with T4 (PDB accession code 2ROX53)(B) Close-up view of one of the two identical T4 binding sites showing a ribbon depicted tetramer (colored by chain) with a “Connelly” molecular surface applied to residues within 8 Å of T4 (hydrophobic = grey, polar = purple). The innermost halogen binding pockets (HBPs) 3 and 3′ are composed of the methyl and methylene groups of Ser117/117′, Thr119/119′, and Leu110/110′. HBPs 2 and 2′ are made up by the side chains of Leu110/110′, Ala109/109′, Lys15/15′, and Leu17/17′. The outermost HBPs 1 and 1′ are lined by the methyl and methylene groups of Lys15/15′, Ala108/108′, and Thr106/106′. These figures were generated using the program MOE (2011.10), Chemical Computing Group, Montreal, Canada. (C) Schematic representation of the T4 binding pocket and the amino acids that are targeted by the substituted (R = CH3 or Cl) aryl ring A connected by linker X (stilbene or 1,3,4-oxadiazole ring linker) to aryl ring B that contains an electrophile Y (sulfonyl fluoride or fluorophenol thioester) for attack by the pKa-perturbed nucleophilic ε-amino group of Lys15.