Table 1.
Substrate Generality for the One-pot Tandem Epoxidation/Rearrangement Reaction.[a]
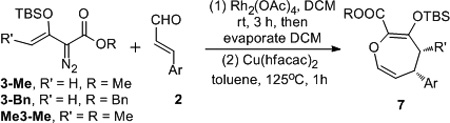 | ||||
|---|---|---|---|---|
| Entry | 3 | 2, Ar | 7 | yield (%)[b] |
| 1 | 3-Me | 4-NO2C6H4 | 7a | 95 |
| 2 | 3-Me | 3-NO2C6H4 | 7b | 95 |
| 3 | 3-Me | 2-NO2C6H4 | 7c | 95 |
| 4 | 3-Me | C6H5 | 7d | 82 |
| 5 | 3-Me | 4-CF3C6H4 | 7e | 95 |
| 6 | 3-Me | 4-BrC6H4 | 7f | 90 |
| 7 | 3-Me | 4-ClC6H4 | 7g | 88 |
| 8 | 3-Me | 4-FC6H4 | 7h | 90 |
| 9 | 3-Me | 4-MeC6H4 | 7i | 81 |
| 10 | 3-Bn | 4-NO2C6H4 | 7j | 95 |
| 11 | 3-Bn | 2-NO2C6H4 | 7k | 95 |
| 12 | 3-Bn | C6H5 | 7l | 85 |
| 13 | Me3-Me | 4-NO2C6H4 | 7m | 95[c] |
The reaction was carried out with 3 (0.36 mmol), aldehyde (0.30 mmol), Rh2(OAc)4 (2 mol%), Cu(hfacac)2 (5 mol%), respective solvent (2 mL).
Isolated yield of 7 after chromatography.
The 1H NMR spectrum of the reaction mixture showed only one diasteroisomer, whose stereochemistry was confirmed by NOe analysis, see SI.
