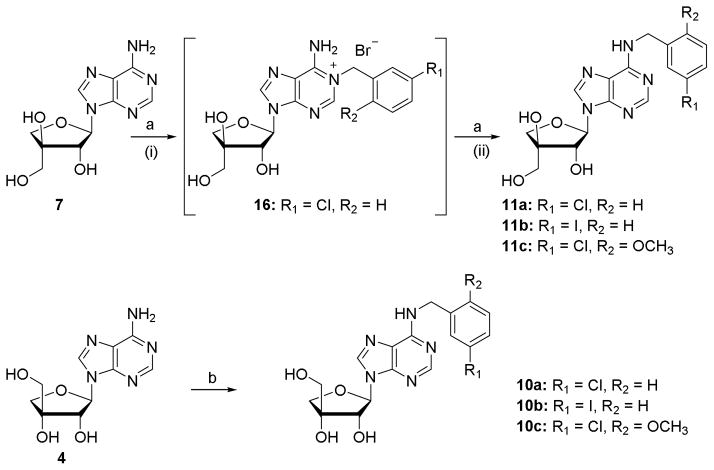
Scheme 1.
Synthesis of N6 substituted apioadenosines. Reagents and conditions: (a) (i) appropriate benzyl bromide, DMF, 50 °C, 48 h; (ii) 25% NH4OH, 50 °C, 48 h or 90 °C, 3 h, 20–56% over two steps; (b) (i) appropriate benzyl bromide, DMF, rt, 48 h; (ii) 25% NH4OH, 50 °C, 24 h, 8–21% over two steps.