Scheme 3.
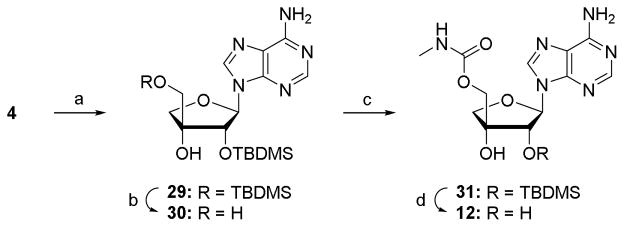
Synthesis of 5′-O-methylcarbamoyl-β-D-apio-D-furanoadenosine. Reagents and conditions: (a) TBDMSCl, imidazole, DMF, rt, 18 h, 74%; (b) TCA-H2O, THF, 0°C, 1 h, rt, 3 h, 35%; (c) (i) CDI, THF, rt, 3 h; (ii) MeNH2, rt, 16 h, 72%; (d) NH4F, MeOH, 50 °C, 48 h, 90%.
