Abstract
Background
Although obesity putatively occurs when individuals consume more calories than needed for metabolic needs, numerous risk factor studies have not observed significant positive relations between reported caloric intake and future weight gain, potentially because reported caloric intake is inaccurate.
Objective
The present study tested the hypothesis that objectively measured habitual energy intake, estimated with doubly labeled water, would show a stronger positive relation to future weight gain than self-reported caloric intake based on a widely used food frequency measure.
Design
253 adolescents completed a doubly labeled water (DLW) assessment of energy intake (EI), a food frequency measure, and a resting metabolic rate (RMR) assessment at baseline, and had their body mass index (BMI) measured at baseline and at 1- and 2-year follow-ups.
Results
Controlling for baseline RMR, elevated objectively measured EI, but not self-reported habitual caloric intake, predicted increases in BMI over a 2-year follow-up. On average, participants under-reported caloric intake by 35%.
Conclusions
Results provide support for the thesis that self-reported caloric intake has not predicted future weight gain because it is less accurate than objectively measured habitual caloric intake, suggesting that food frequency measures can lead to misleading findings. However, even objectively measured caloric intake showed only a moderate relation to future weight gain, implying that habitual caloric intake fluctuates over time and that it may be necessary to conduct serial assessments of habitual intake to better reflect the time-varying effects of caloric intake on weight gain.
Keywords: Caloric intake, doubly labeled water, prospective, weight gain
INTRODUCTION
It is widely accepted that obesity results from a positive energy balance that occurs when individuals consume more calories than required for basal metabolic needs and physical activity (Hall et al., 2012). Although controlled experiments have established that elevated caloric intake results in subsequent weight gain in both animals and humans (e.g., Pearcey & Castro, 2002; Lissner, Levitsky, Strupp, Kalkwarf, & Roe, 1987; Warwick & Schiffman, 1992), prospective obesity risk factor studies typically have not found a significant relation between elevated self-reported caloric intake and future weight gain in humans (e.g., Berkey et al., 2000; Chaput et al., 2009; Klesges, Isbell, & Klesges, 1992; Maffeis, Talamini, & Tato, 1998). These null findings have prompted various alternative explanations for weight gain, such as the thesis that a low metabolic rate causes obesity (Hall et al., 2012). However, an alternative explanation is that self-reported caloric intake is inaccurate. Numerous studies have established that people usually under-report caloric intake by varying degrees, particularly those with elevated body mass or high dietary restraint scores (e.g., Sawaya et al., 1996; Bandini, Schoeller, Dry, & Dietz, 1990; Lichtman et al., 1992; Stice, Cooper, Schoeller, Tappe, & Lowe, 2007; Black & Cole, 2001). Energy intake estimated from doubly labeled water (DLW) is a more accurate measure of energy intake (EI) than self-report dietary intake measures, such as food frequency questionnaires and 24-hr dietary recall interviews. However, very limited data exists testing the relation between DLW estimated EI and future weight gain. We were able to locate only one prior study that tested the predictive effects of EI from doubly labeled water on weight gain, reporting a significant positive relation (Tataranni et al., 2003). The Tatarranni et al, study also found that a low resting metabolic rate (RMR) predicted future weight gain, but not DLW estimated energy expenditure (EE). However, it appears that no study has compared the predictive relation of DLW estimated caloric intake versus that of self-reported caloric intake to future weight gain. Accordingly, the goal of this report is to test whether EI estimated from DLW shows a stronger relation to future objectively measured weight gain, relative to the effects of self-reported habitual caloric intake, as assessed by a commonly used food frequency measure. In our predictive models, we controlled for baseline RMR to adjust for individual differences in metabolic needs across subjects.
SUBJECTS AND METHODS
Participants
The sample consisted of 253 participants recruited from two studies (See Table 1 for sample characteristics). 162 of these participants were lean adolescents recruited from local high schools (51% female, M age = 15.32) for a study on neural vulnerability factors that predict future weight gain, and 91 were college-aged females recruited from a local university who were randomly selected from a larger study of young women with body image concerns (M age = 18.42) who had enrolled in an obesity prevention trial. The overall sample consisted of 2% African American, 2% Asian, 85% European Americans, 4% American Indian and Alaska Native participants, 1% Native Hawaiian or other Pacific Islander, and 6% other or mixed racial heritage. Exclusion criteria included pregnancy, diabetes, conditions requiring supplemental oxygen, or current DSM-IV anorexia nervosa, bulimia nervosa, or binge eating disorder. Participants (and parents of those who were minors) provided informed written consent. Participants provided data during four visits to the lab: baseline (T1), 2 weeks after baseline (T2), 1 year after baseline (T3), and 2 years after baseline (T4). At baseline, participants arrived at the laboratory after an overnight fast to complete the first DLW assessment. They then returned 2 weeks later for the follow-up DLW assessment. They were also required to avoid traveling more than 200 miles from the study site in the 2-weeks between T1 and T2 due to regional differences in levels of naturally occurring elements found in drinking water (deuterium and oxygen-18) that can affect the levels in the DLW isotope used to calculate TEE. Twenty-three (9%) participants did not complete the 1-year follow-up assessment, and fourteen (6%) did not complete the 2-year follow-up assessment.
Table 1.
| Mean ± SD3 | Minimum | Maximum | |
|---|---|---|---|
| Baseline age | 16.5 ± 1.75 | 14 | 20 |
| Baseline BMI | 21.87 ± 3.2 | 16.7 | 43.7 |
| 1-year BMI | 22.38 ± 3.6 | 16.7 | 48.4 |
| 2-year BMI | 22.60 ± 3.4 | 17.1 | 44.7 |
| Baseline RMR (kcal) | 1384 ± 261 | 684 | 2165 |
| DLW EI (kcal) | 2572 ± 755 | 884 | 6330 |
| Self-report EI (kcal) | 1661 ± 801 | 339 | 5295 |
n = 253
Data presented is untransformed.
Acronyms: SD = standard deviation, BMI = body mass index, DLW = doubly labeled water, RMR = resting metabolic rate, EI= energy intake
Measures
Body Mass
The BMI (kg/m2) was used to reflect height-adjusted adiposity. After removal of shoes and coats, height was measured to the nearest millimeter using a stadiometer and weight was assessed to the nearest 0.1 kg using a digital scale. Two measures of height and weight were obtained and averaged at baseline and at 1- and 2-year follow-ups. BMI correlates with direct measures of total body fat such as dual energy X-ray absorptiometry (r = 0.80 to .90) and with health measures including blood pressure, adverse lipoprotein profiles, atherosclerotic lesions, serum insulin levels, and diabetes mellitus in adolescent samples (Dietz & Robinson, 1998).
Energy Intake
DLW was used to estimate EI over a 2-wk period. DLW provides a very accurate measure of intake that is immune to biases associated with dietary recalls or diet diaries (Schutz, Weinsier, & Hunter, 2001; Johnson, 2002). DLW uses isotopic tracers to assess total carbon dioxide production, which can be used to accurately estimate habitual caloric expenditure (Schoeller et al., 1986). DLW was administered immediately after subjects tested negatively for pregnancy (if applicable). Doses were 1.6 –2.0 g H218O (10 atom percent)/kg estimated total body water. Spot urine samples were collected immediately before DLW was administered and 1, 3, and 4-h postdosing. Two-weeks later, 2 additional spot urine samples were collected at the same time of day as 3- and 4-h post dosing samples. No samples were the first void of the day. Energy expenditure (EE) was calculated by using equation A6 (Schoeller et al., 1986), dilution space ratios (Racette et al., 1994), and the modified Weir 's equation (Weir, 1949) as previously described (Black, Prentice, & Coward, 1986). EI per day was calculated from the sum of EE from DLW and the estimated change in body energy stores from serial body weight measurements performed at baseline (T1) and 2-wk after dosing (T2). This figure was divided by the number of days between T1 and T2 to calculate the daily source of energy substrates from weight loss or storage of excess EI as weight gain (Forbes, 2000). The equation used for each participant was: EI = EE + [(T2 weight – T1 weight) × 7800)] / (T2 date – T1 date). The 7800 kcal/kg is an estimate of the energy density of adipose tissue (Poehlmen, 1989).
Self-reported energy intake
The Block Food Frequency Questionnaire (Block & Subar, 1992) assessed frequency of consumption of specific food types over the past two weeks. Participants are given a definition of a medium portion and asked to indicate the frequency of consumption over the previous 2-wk period. Responses to the question were on a 6-point Likert scale, where 1 = “never in the previous 2-wk period ” to 6 = “daily or more in the previous 2-wk period. ” BFFQ values correlated (r = 0.57) with 4-d food record estimates for total energy intake and most nutrients (24) and showed 2-wk test-retest reliability (mean r = 0.69) (Klohe, et al., 2005).
Statistical Analyses
Multiple imputation was used to replace missing values following best-practice recommendations (Graham, 2009). Missing data were imputed with the Amelia package of the R project (Honaker & King, 2010). The observed and imputed data were compared to ensure they showed similar distributions (Abayomi, Gelman, & Levy, 2008). Missing data were replaced with imputed data and were analyzed separately. Model parameters and standard errors, which incorporate within and between model parameter variability, were combined following Rubin (1987).
We examined the distribution of variables and evaluated potential sources of non-independence. Condition (obesity prevention intervention participants and non-intervention adolescents) and baseline resting metabolic rate (RMR), as measured through indirect calorimetry (see Stice, Durant, Burger, & Schoeller, 2001), were used as a control variables.
Linear mixed effects models, which accommodate multilevel data structures and unevenly spaced longitudinal data (Pinheiro & Bates, 2013), were used to test whether DLW measured EI and self-reported EI predicted increases in BMI at 1- and 2-year follow-up. The Singer and Willett model building sequence was used (Stice, Rohde, Shaw, & Marti, 2013).
RESULTS
In our preliminary analyses, 1- and 2-year BMI was distributed normally, so no transformations were made to these outcomes. A log-transformed version of time was used in all models based on prior analyses with the same data set (Stice et al., 2013; Stice, Rohde, Shaw, & Marti, 2012) in which it was determined that log-transformed time was the best fit for the longitudinal component of the model. Participant condition was coded using two dummy vectors, one for either the college-aged women from the obesity prevention intervention or the non-intervention adolescent participants, and one for the brochure control participants. Table 1 provides means and SD for outcomes at each time point across conditions. Data were complete at baseline, 9% were missing at 1-year follow-up, and 6% were missing at 2-year follow-up. All outcomes were modeled as two-level models in which time points were nested within individuals.
Preliminary analyses compared participants above and below the baseline median BMI score on RMR, DLW EI and self-reported EI at baseline. The high BMI RMR (M = 1433.1, SD = 267.5, range = [684.1, 2165.4]) was greater than the low BMI RMR (M = 1335.3, SD = 246.8, range = [718.3, 1936.0]) (t[250] = 3.02, p = .003); the high BMI DLW EI (M = 2663.1, SD = 790.1, range = [1081, 6330]) was marginally greater than the low BMI DLW EI (M = 2478.8, SD = 708.0, range = [884, 4270]) (t[240] = 1.91, p = .057); and the high BMI self-reported EI (M = 1501.2, SD = 681.1, range = [338.7, 3906.8]) was greater than the low BMI self-reported EI (M = 1818.9, SD = 878.9, range = [414.6, 5294.8]) (t[228] = 3.06, p = .002).
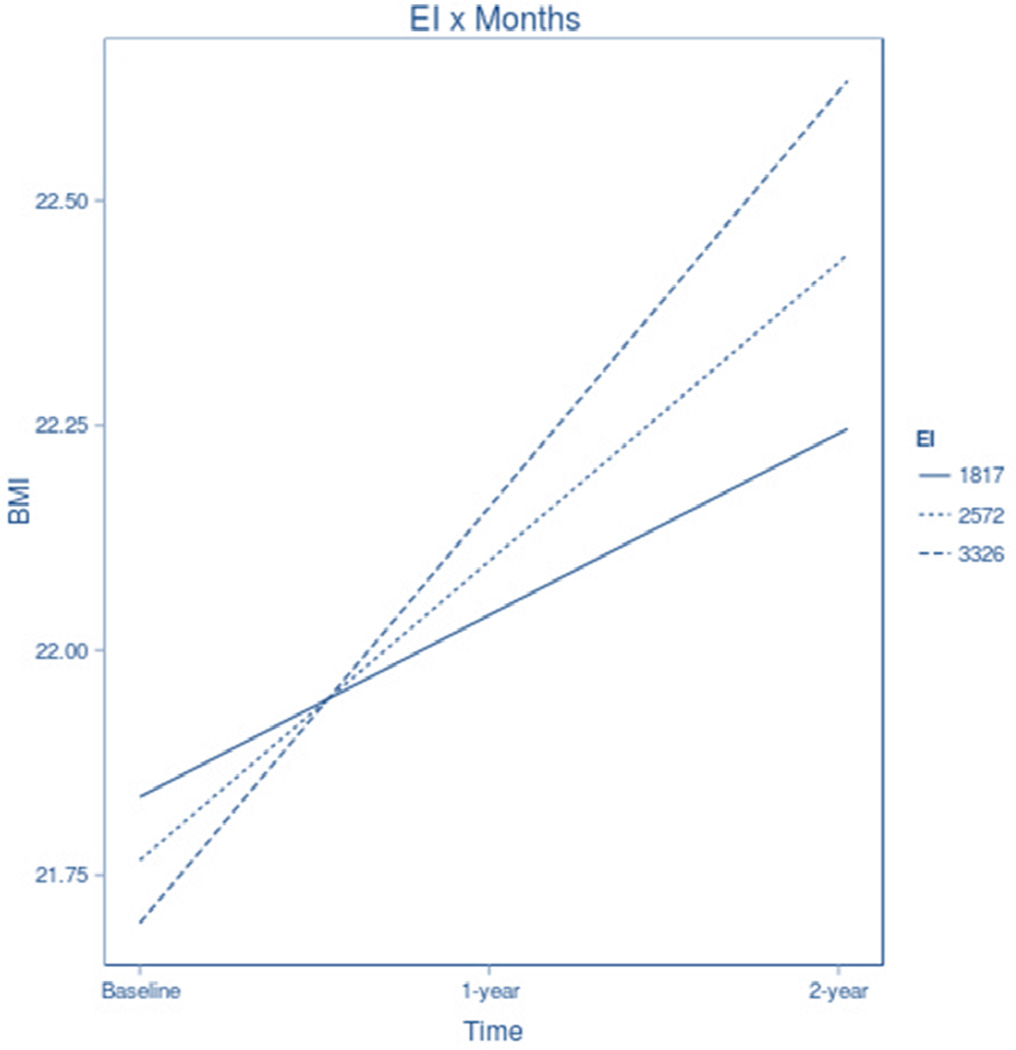
Results addressing the central hypothesis are presented in Table 2. After adjusting for baseline RMR, age, and condition, DLW estimated EI significantly predicted future increases in BMI over the 2-year follow-up period (t (224) = 2.75, p = .006, r = .18; see Figure 1). Also consistent with expectations, self-reported EI did not show a significant relation to future increases in BMI over this time period (t (217) = .933, p = .356, r = .06).
Table 2.
Effects for BMI change over time.
| Moderator | Parameter | Coefficient | SE | df | t | p | r |
|---|---|---|---|---|---|---|---|
| Baseline RMR | Intercept | 2.02 | 1.18 | 223 | 1.71 | .089 | |
| Baseline BMI | 0.97 | 0.02 | 240 | 47.64 | <.001 | ||
| Months1 | −0.32 | 0.15 | 234 | −2.12 | .035 | ||
| RMR | 0.00 | 0.00 | 241 | 0.18 | .855 | ||
| Months1 × RMR | 0.00 | 0.00 | 231 | 3.53 | <.001 | .23 | |
| DLW EI | Intercept | 1.49 | 1.16 | 220 | 1.29 | .200 | |
| Baseline BMI | 0.97 | 0.02 | 240 | 47.8 | <.001 | ||
| Months1 | −0.06 | 0.10 | 228 | −0.63 | .532 | ||
| DLW EI | −0.00 | 0.00 | 234 | −0.91 | .362 | ||
| Baseline age | −0.11 | 0.06 | 217 | −1.76 | .080 | ||
| RMR | 0.00 | 0.00 | 225 | 2.5 | .013 | ||
| Months1 × DLW EI | 0.00 | 0.00 | 224 | 2.75 | .006 | .18 | |
| Self-reported EI | Intercept | 1.02 | 1.15 | 222 | 0.89 | .376 | |
| Baseline BMI | 0.97 | 0.02 | 241 | 47.18 | <.001 | ||
| Months1 | 0.15 | 0.07 | 227 | 2.3 | .023 | ||
| Baseline age | −0.09 | 0.06 | 222 | −1.54 | .124 | ||
| RMR | 0.00 | 0.00 | 230 | 3.67 | <.001 | ||
| Self-reported EI | −0.00 | 0.00 | 234 | −1.51 | .133 | ||
| Months1 × self-reported EI | 0.00 | 0.00 | 217 | 0.93 | .356 | .06 |
log transformed
Figure 1.
Simple slopes of the regression of the predicted level of BMI at baseline, 1-year, and 2-year follow-up at high, medium, and low levels of EI. Note. High, medium, and low values of EI are defined as plus and minus 1 SD about the mean (M = 2571.72, SD = 754.68).
DISCUSSION
As hypothesized, objectively measured habitual caloric intake, as estimated using DLW showed a significant positive relation to future increases in BMI, whereas self-reported caloric intake did not. Thus, results accord with the thesis that the lack of predictive effects in past obesity risk factor studies was due to the use of self-report measures of dietary intake, which are known to underestimate habitual caloric intake. The discrepancy between objectively measured and reported dietary intake was very large; whereas the average participants reported consuming 1661 kcals daily, the DLW estimated daily caloric intake was 2572 (which translates into an average under-reporting of daily caloric intake of 35.4%; range = 13%-246%, SD = 38%). The large discrepancy between self-reported and DLW estimated energy intake resulted in a very low correlation between the two measures (r = .14), clearly indicating that food frequency measures are very inaccurate, accounting for less than 2% of the variance in objectively measured caloric intake in adolescents in the present sample.
Also of note, the relation between objectively measured habitual caloric intake and future weight gain was only a moderate effect size. The relatively small effect may occur because caloric intake fluctuates over time, which limits the predictive validity of habitual intake from only a 2-week observational period. Indeed, there is evidence that weight gain often occurs more over the holidays and on weekends versus on weekdays (Cook, Subar, Troiano, & Schoeller, 2012), suggesting that it might be necessary to collect serial measures of objectively measured caloric intake to more accurately predict future weight gain based on caloric intake.
Given that a positive energy balance occurs because individuals are consuming more calories than required for basal metabolic needs and physical activity, it is interesting that DLW estimated energy expenditure, which is a key term in the equation used to estimate habitual energy intake, has shown mixed findings as to whether it predicts future weight gain. Although some studies have not found a significant relation between EE and future weight gain (Tataranni et al., 2003; Cook et al, 2012; Stunkard, Berkowitz, Stallings, & Schoeller, 1999; Goran et al., 1998; Luke et al., 2009), others have shown a positive relation between EE and future weight gain, indicating that higher, rather than lower EE is related to weight gain (Goran et al., 1998; Schoeller, 2008; Stunkard, Berkowitz, Schoeller, Maislin, & Stallings, 2004; Luke et al., 2007). In our sample, there was a significant positive correlation between DLW estimated EE and EI (r = .71), and when EE is entered into the same linear mixed effects model instead of EI, it showed a significant positive relation to future weight gain (t(202) = 2.10, p = .04, r = .15).
It is important to consider the limitations of the current study. First, the sample contained a limited number of individuals from ethnic and racial minority groups, suggesting that results should be generalized with caution. Second, our sample focused on late adolescents, so future research will be necessary to determine whether similar effects emerge for children and adults. Third, we only used one measure of self-reported caloric intake in this study, and future studies should test the accuracy of additional methods of collecting self-reported intake data, such as 24-hr dietary recalls.
These present results provide support for the widely accepted energy balance model of obesity and suggest that self-reported caloric intake is so inaccurate that it might not be worth collecting these data in future studies, at least using food frequency measures like the one utilized herein. An important direction for future research will be to develop alternative procedures for objectively measuring dietary intake, which will be vital for obesity risk factor studies and for obesity prevention and treatment trials. Moreover, the fact that even objectively measured caloric intake showed only a modest relation to future weight gain suggests that it may be necessary to collect multiple measures of habitual energy intake to better model the time-varying changes in energy intake that presumably drives weight gain.
Self-reported measures of caloric intake have questionable accuracy.
Objectively measured caloric intake may be a more accurate measure.
ACKNOWLEDGMENTS
Eric Stice was responsible for study design and contributed to data analysis and manuscript writing and revisions. Shelley Durant contributed to data analysis, manuscript writing and revisions, and participated in data collection. The authors thank Dale Schoeller for serving as a consultant for these projects and conducting the DLW analyses, as well as C. Nathan Marti for assistance with the statistical modeling.
Support for this work was provided by NIH R01 DK072932 and R01 DK080760
Abbreviations
- DLW
doubly labeled water
- EI
energy intake
- RMR
resting metabolic rate
- BMI
body mass index
- EE
energy expenditure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifiers: NCT00433680 and NCT02084836
The authors declare no conflicts of interest.
REFERENCES
- Abayomi K, Gelman A, Levy M. Diagnostics for multivariate imputations. J R Stat Soc. 2008;57:273–291. [Google Scholar]
- Bandini LG, Schoeller DA, Dyr HN, Dietz WH. Validity of reported energy intake in obese and nonobese adolescents. Am J Clin Nutr. 1990;52:421–425. doi: 10.1093/ajcn/52.3.421. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Rockett HR, Field AE, Gillman MW, Frazier AL, Camargo CA, Colditz GA. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics. 2000;105:1–9. doi: 10.1542/peds.105.4.e56. [DOI] [PubMed] [Google Scholar]
- Black AE, Cole T. Biased over- or under-reporting is characteristic of individuals whether over time or by different assessment methods. J Am Diet Assoc. 2001;101:70–80. doi: 10.1016/S0002-8223(01)00018-9. [DOI] [PubMed] [Google Scholar]
- Black AE, Prentice AM, Coward WA. Use of food quotients to predict respiratory quotients for the doubly-labeled water method of measuring energy-expenditure. Hum Nutr Clin Nutr. 1986;40:381–391. [PubMed] [Google Scholar]
- Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire—the 1987 National-Health Interview Survey. J Am Diet Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- Chaput J, Leblanc C, Perusse L, Despres J, Bouchard C, Tremblay A. Risk factors for adult overweight and obesity in the Quebec Family Study: Have we been barking up the wrong tree? Obesity. 2009;17:1964–1970. doi: 10.1038/oby.2009.116. [DOI] [PubMed] [Google Scholar]
- Cook C, Subar A, Troiano R, Schoeller D. Relation between holiday weight gain and total energy expenditure among 40- to 69-y-old men and women (OPEN study) Am J Clin Nutr. 2012;95:726–731. doi: 10.3945/ajcn.111.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH, Robinson TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- Forbes GB. Body fat content influences the body composition response to nutrition and exercise. In: Yasumura S, Wang J, Pierson RN, editors. In vivo body composition studies. New York, NY: New York Acad Sciences; 2000. pp. 359–365. [DOI] [PubMed] [Google Scholar]
- Goran M, Shewchuk R, Gower B, Nagy TR, Carpenter WH, Johnson RK. Longitudinal changes in fatness in white children: No effect of childhood energy expenditure. Am J Clin Nutr. 1998;67:309–316. doi: 10.1093/ajcn/67.2.309. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hall K, Heymsfield S, Kemnitz J, Klein S, Schoeller D, Speakman J. Energy balance and its components Implications for body weight regulation. Am J Clin Nutr. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaker J, King G. What to do about missing values in time-series cross-section data. Am J Pol Sci. 2010;54:561–581. [Google Scholar]
- Johnson RK. Dietary intake-how do we measure what people are really eating? Obes Res. 2002;10(suppl 1):63S–8S. doi: 10.1038/oby.2002.192. [DOI] [PubMed] [Google Scholar]
- Klesges R, Isbell T, Klesges L. Relationship between dietary restraint, energy intake, physical activity, and body weight: A prospective analysis. J Abnorm Psychol. 1992;101:668–674. doi: 10.1037//0021-843x.101.4.668. [DOI] [PubMed] [Google Scholar]
- Klohe DM, Clarke KK, George CC, Milani TJ, Hanss-Nuss H, Freeland-Graves J. Relative validity and reliability of a food frequency questionnaire for a triethnic population of 1-year-old to 3-year-old children for low income families. J Am Diet Assoc. 2005;105:727–734. doi: 10.1016/j.jada.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, Weisel H, Heshka S, Matthews DE, Heymsfield SB. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327:1893–1898. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- Lissner L, Levitsky DA, Strupp BJ, Kalkwarf HJ, Roe DA. Dietary fat and the regulation of energy intake in human subjects. Am J Clin Nutr. 1987;46:886–892. doi: 10.1093/ajcn/46.6.886. [DOI] [PubMed] [Google Scholar]
- Luke A, Dugas L, Ebersole K, Durazo-Arvizu R, Cao G, Schoeller D, Adeyemo A, Brieger W, Cooper R. Energy expenditure does not predict weight change in either Nigerian or African American women. Am J Clin Nutr. 2009;89:169–176. doi: 10.3945/ajcn.2008.26630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A, Durazo-Arvizu RA, Cao G, Forrester TE, Wilks RJ, Schoeller DA, et al. Activity, adiposity and weight change in Jamaican adults. West Indian Med J. 2007;56:398–403. [PubMed] [Google Scholar]
- Maffeis C, Talamini G, Tato L. Influence of diet, physical activity and parents’ obesity on children’s adiposity: A four-year longitudinal study. Int J Obes. 1998;22:758–764. doi: 10.1038/sj.ijo.0800655. [DOI] [PubMed] [Google Scholar]
- Pearcey S, Castro J. Food intake and meal patterns of weight-stable and weight-gaining persons. Am J Clin Nutr. 2002;76:107–112. doi: 10.1093/ajcn/76.1.107. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. nlme (Version 3.1e108) [Computer program and manual] Retrieved January 16, 2013, from http://cran.r-project.org/web/packages/nlme/nlme.pdf.
- Poehlmen ET. A review: exercise and its influence on resting metabolic energy metabolism in man. Med Sci Sports Exerc. 1989;21:515–525. [PubMed] [Google Scholar]
- Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of h-2-labeled and o-18-labeled water in humans. Am J of Physiol. 1994;267:E585–E590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- Sawaya A, Tucker K, Tsay R, Walter W, Saltzman E, Dallal G, Roberts S. Evaluation of four methods for determining energy intake in young and older women: comparison with doubly labeled water measurements of total energy expenditure. Am J Clin Nutr. 1996;63:491–499. doi: 10.1093/ajcn/63.4.491. [DOI] [PubMed] [Google Scholar]
- Schoeller DA. Insights into energy balance from doubly labeled water. Int J Obes. 2008;32:S72–S75. doi: 10.1038/ijo.2008.241. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy-expenditure by doubly labeled water - validation in humans and proposed calculation. Am J Physiol. 1986;250:R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- Schutz Y, Weinsier RL, Hunter GR. Assessment of free-living physical activity in humans: an overview of currently available and proposed new measures. Obes Res. 2001;9:368–379. doi: 10.1038/oby.2001.48. [DOI] [PubMed] [Google Scholar]
- Stice E, Cooper JA, Schoeller DA, Tappe K, Lowe MR. Are dietary restraint scales valid measures of moderate- to long-term dietary restriction? Objective biological and behavioral data suggest not. Psychol Assess. 2007;19:449–458. doi: 10.1037/1040-3590.19.4.449. [DOI] [PubMed] [Google Scholar]
- Stice E, Durant S, Burger KS, Schoeller D. Weight suppression and risk of future increases in body mass: effects of suppressed resting metabolic rate and energy expenditure. Am J Clin Nutr. 2001;94:7–11. doi: 10.3945/ajcn.110.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Shaw H, Marti CN. Efficacy trial of a selective prevention program targeting both eating disorders and obesity among female college students: 1- and 2-year follow-up effects. J Con Clin Psychol. 2013;81:183–189. doi: 10.1037/a0031235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Shaw H, Marti CN. Efficacy Trial of a Selective Prevention Program Targeting Both Eating Disorder Symptoms and Unhealthy Weight Gain Among Female College Students. J Con Clin Psychol. 2012;80:164–170. doi: 10.1037/a0026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G, Stallings VA. Predictors of body size in the first 2 y of life: a high-risk study of human obesity. Int J Obes Relat Metab Disord. 2004;28:503–513. doi: 10.1038/sj.ijo.0802517. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr. 1999;69:524–530. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- Tataranni P, Harper I, Snitker S, Del Parigi A, Vozarova B, Bunt J, Bogardus C, Ravussin E. Body weight gain in free-living Pima Indians: Effect of energy intake vs expenditure. Int J Obes Relat Metab Disord. 2003;27:1578–1583. doi: 10.1038/sj.ijo.0802469. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Schiffman SS. Role of dietary fat in calorie intake and weight gain. Neurosci Biobeh Rev. 1992;16:585–596. doi: 10.1016/s0149-7634(05)80198-8. [DOI] [PubMed] [Google Scholar]
- Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]