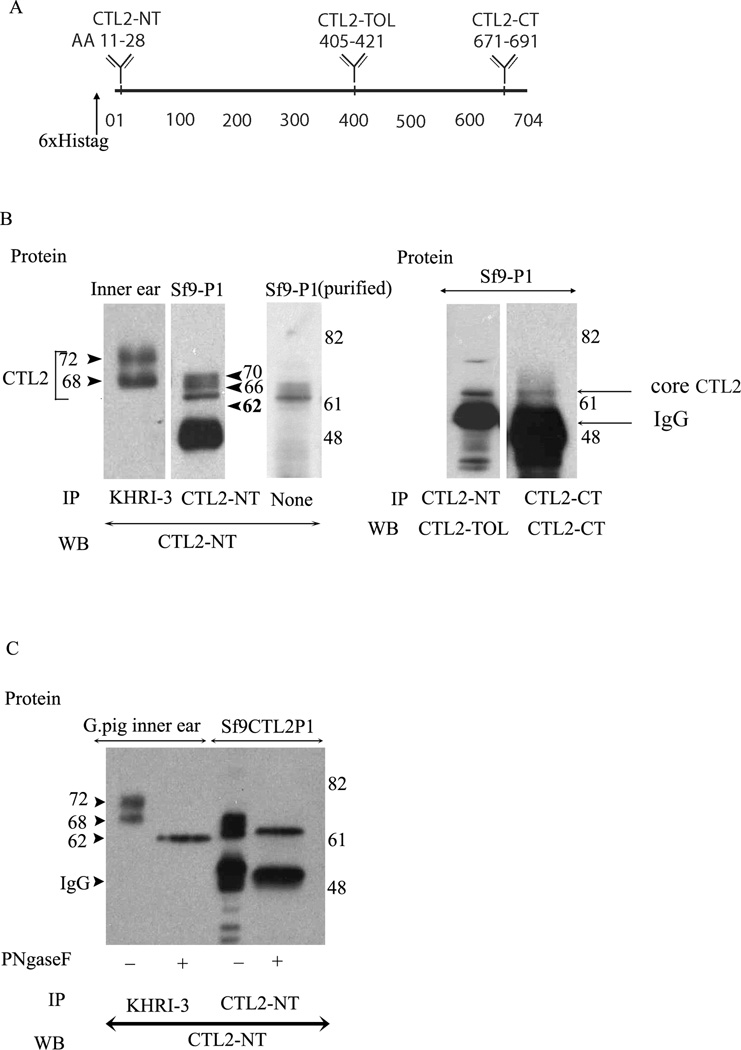
Figure 4.
Immunoprecipitation and western blot comparative analysis of the mature and de-glycosylated forms of guinea pig inner ear Sf9 produced CTL2. Panel A. Schematic representation of CTL2 protein showing the location of the N-terminus His-tag (6×) and location of the three antigenic peptides used to raise rabbit antibodies. CTL2-NT was raised to a 19 amino acid peptide in the N-terminal domain of human CTL2. CTL2-TOL was raised against 17 amino acid peptide in the third outer loop. CTL2-CT was made against a 20-amino acid antigenic peptide located near the C-terminus. Panel B. Comparison of CTL2 from guinea pig to insect cell-produced rHuCTL2. left panel: immunoprecipitation and western blot of CTL2 from guinea pig inner ear tissue (Lane 1), rHuCTL2 P1 from Sf9 insect cells (lane 2). Lane 3 shows Nickel -NTA column purified rHuCTL2 P1 probed with CTL2-NT antibody. Lane 4: rHuCTL2- P1 precipitated from Sf9 cells with CTL2-NT and probed with CTL2-TOL. Lane 5: rHuCTL2 P1 precipitated from Sf9 cells with CTL2-CT antibody and probed with CTL2-CT. Both antibodies bind to the un-glycosylated 62 kDa core protein. Panel C. Deglycosylation of native CTL2 and rHuCTL2-P1. Immunoprecipitated CTL2 from guinea pig inner ear or rHuCTL2 from Sf9 cells was treated with PNGase F. Lane 1: Untreated guinea pig CTL2. Lane 2: deglycosylated guinea pig CTL2. Lane 3: glycosylated rHuCTL2-P1. Lane 4: Deglycosylated rHuCTL2-P1. The difference in the molecular mass between native and recombinant deglycosylated protein, is due to the histidine tag and linker. Both guinea pig and rHuCTL2 have a core protein of ~62 kDa.