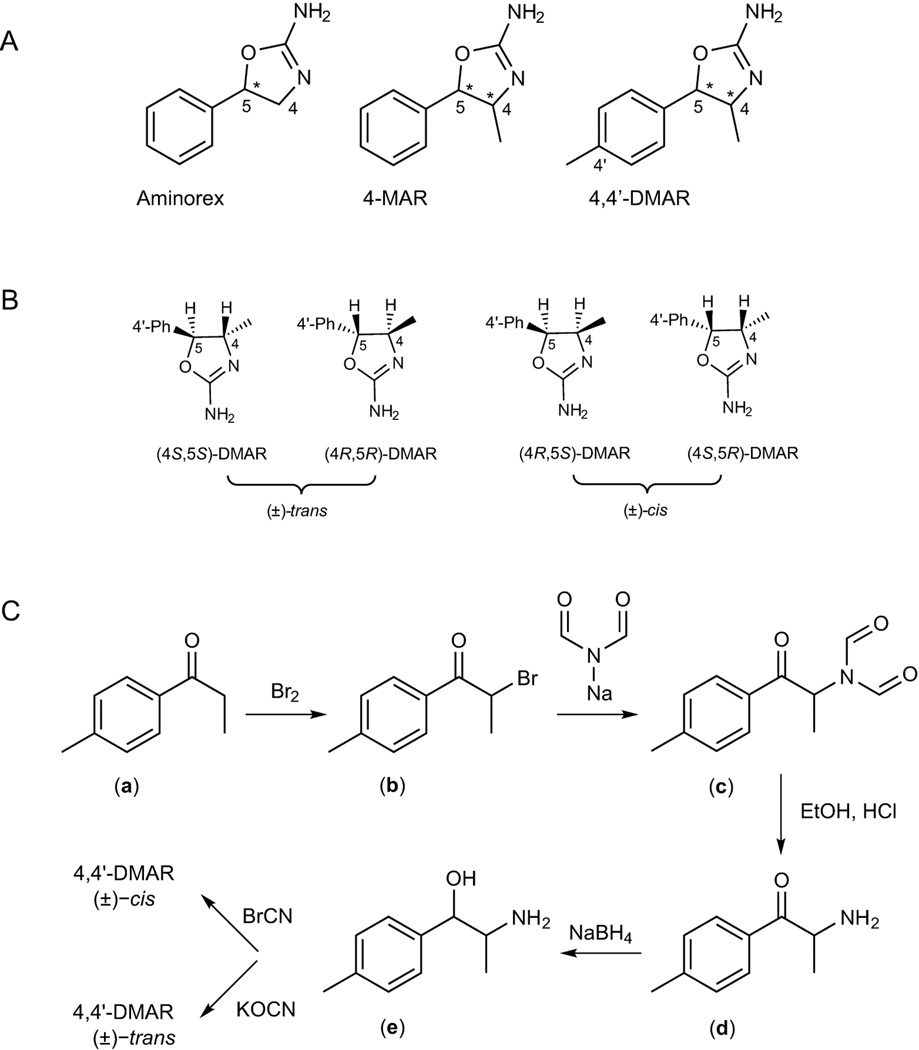
Figure 1.
A: Chemical structures of the three psychostimulants aminorex, 4-methylaminorex (4-MAR) and para-methyl-4-methylaminorex (4,4'-DMAR). B: structural representations of all four existing 4,4'-DMAR enantiomers; 4'-Ph represents the para-substituted phenyl ring. C: synthetic route to both (±)-cis- and (±)-trans-4,4'-DMAR. Both isomers were prepared from the same 4'-methylnorephedrine precursor (e) using cyanogen bromide or potassium cyanate to yield the (±)-cis- and (±)-trans product, respectively.
214×246mm (300 × 300 DPI)