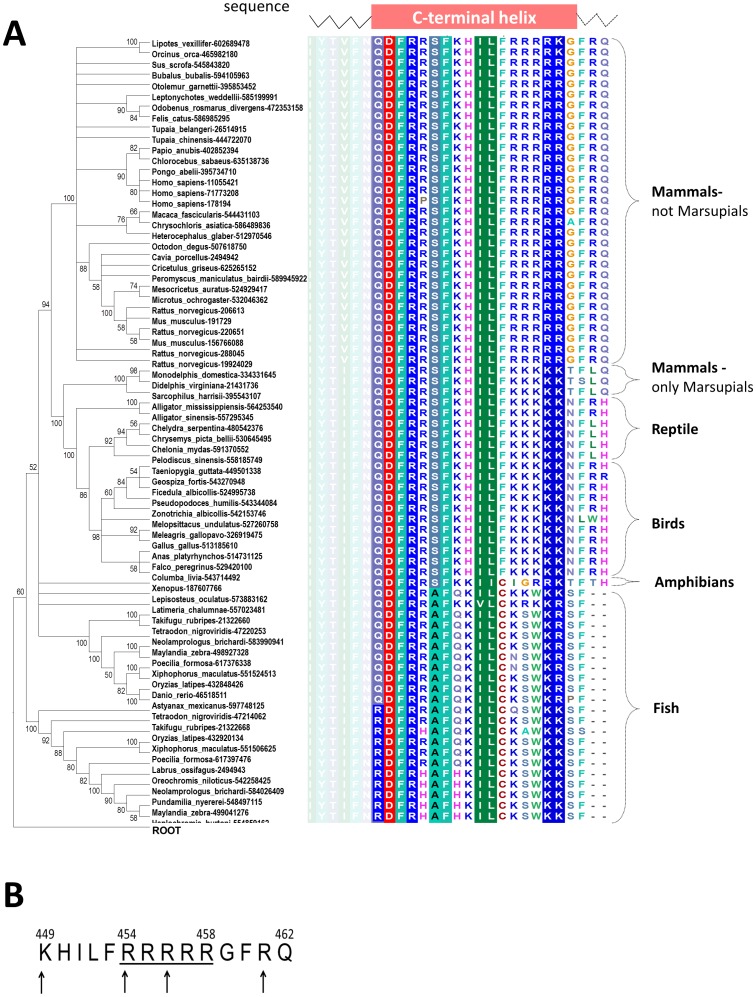
Figure 2. The Minimum evolution tree and multiple sequence alignment of C-terminal tail of the α2C-adrenoceptor family.
Panel A - proteins are indicated by the species name and the NCBI GI number. Values at the nodes indicate the statistical support for the particular branches, according to the bootstrap test. For each protein also its C-terminal sequence is presented. Sequences were aligned by MUSCLE program. Amino acids are colored according to the chemical properties of their side-chains (negatively charged: red, positively charged: blue, polar: magenta, hydrophobic: green. Only the alignment that corresponds to the C-terminal helix and flanking residues is shown. The helix was predicted by GeneSilico metaserver. Panel B - the last 14 amino acids of a2C-AR C-terminus highlighting the arginine-rich stretch (underlined). This region is conserved in mammals and in human arteriole-derived vascular smooth muscle cells (microVSM) interacts with the actin-binding protein filamin-2, shown in experimental studies to be necessary for receptor translocation to the cell surface. The numbers denote amino acids in the full-length a2C-AR polypeptide. The arrows point to amino acid residues identified by in-silico modeling to be involved in interaction with filamin-2.