Figure 12.
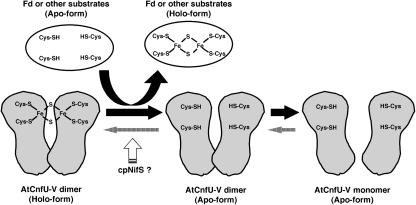
Model of Scaffold Function of AtCnfU-V in Iron-Sulfur Cluster Transfer to Apo-Proteins.
AtCnfU-V exists as a [2Fe-2S]-containing dimer. The iron-sulfur cluster is assembled intermolecularly between the two identical N-terminal domains in the dimer. The cluster can be transferred to certain substrate apoproteins, including ferredoxin (Fd). Upon the transfer, the holo-AtCnfU-V dimer is converted to the apo-dimer or further to apo-monomers. The new [2Fe-2S] cluster is then recharged on the apo-dimeric form of AtCnfU-V, possibly with the aid of the chloroplast-localized NifS-like protein, cpNifS, as a sulfur donor.