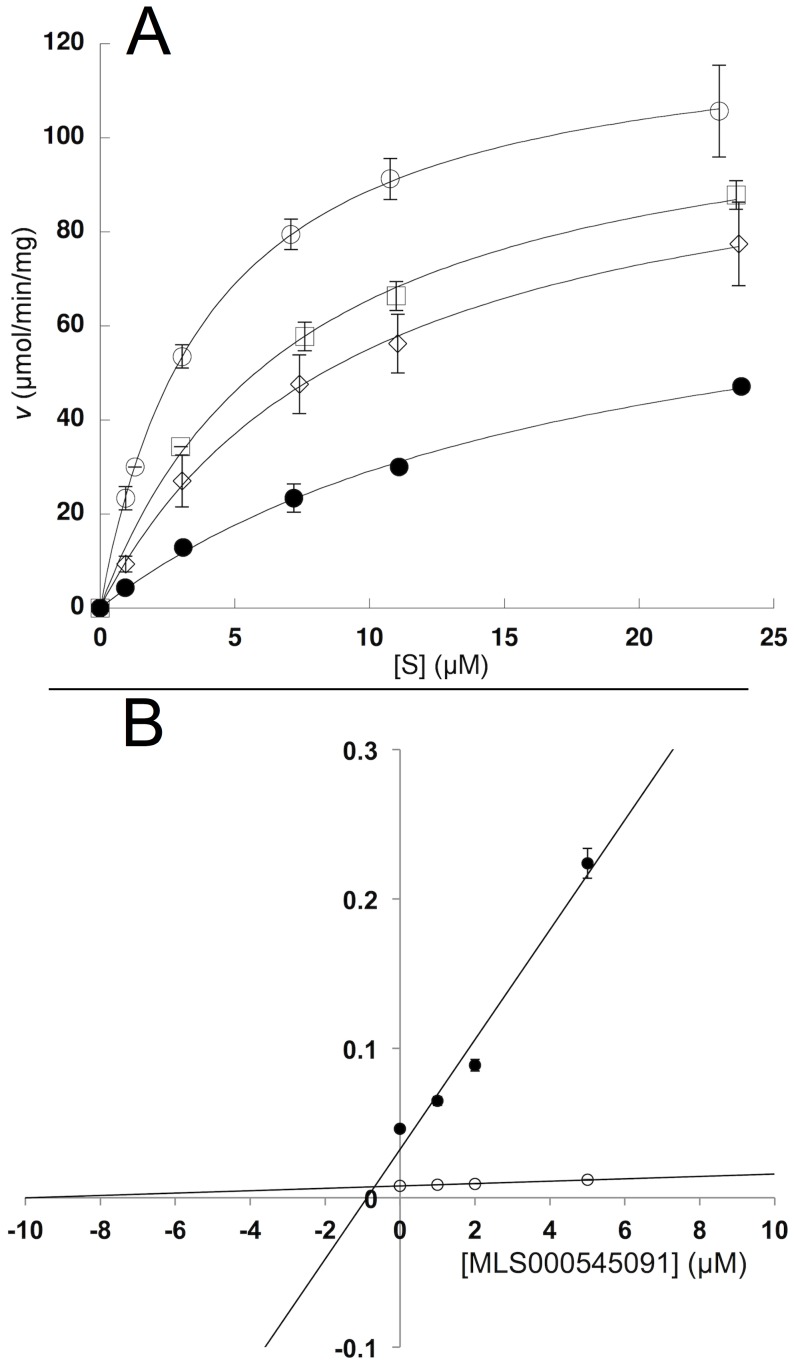
Figure 4. Steady-state kinetics data for the determination of Ki and Ki′ for 15-LOX-2 with MLS000545091.
(A) Initial enzymatic rate (µmol/min/mg) versus substrate concentration (µM) at inhibitor concentrations of 0 µM (open circles) 1 µM (open squares) 2 µM (open diamonds) and 5 µM (closed circles) fitted to the Henri–Michaelis–Menten equation to yield V max (µmol/min/mg) and V max/K M (mol/min/mg/µM). All measurements were done in triplicate. (B) KM/Vmax replot (closed circles) (units are µM/µmol/min/mg) versus [Inhibitor] (µM), which yielded a Ki of 0.9+/−0.4 µM. 1/Vmax replot (open circles) (units are 1/µmol/min/mg) versus [Inhibitor] (µM), which yielded a Ki′ of 9.9+/−0.7 µM, indicating weak mixed-type inhibition.