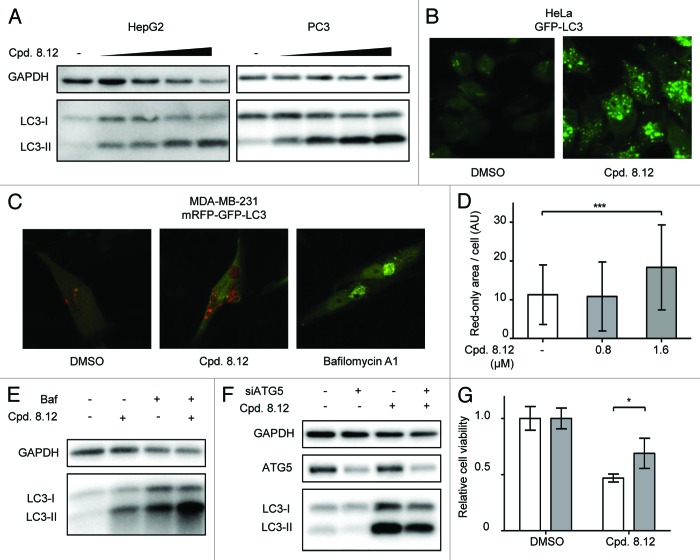
Figure 3. Compound 8.12 increases cellular autophagy. (A) HepG2 and PC3 cells were treated with either vehicle only, 0.2, 0.4, 0.8, and 1.6 µM (HepG2) or 0.4, 0.8, 1.6, and 2.0 µM (PC3) of compound 8.12 for 48 h, whereupon cells were harvested and lysates subject to immunoblot analysis for LC3-II levels to detect autophagy induction. (B) HeLa cells stably expressing GFP-tagged LC3 were treated with vehicle only or 1.6 µM of compound 8.12 for 48 h, whereupon green fluorescent puncta were visualized by confocal microscopy. (C) MDA-MB-231 cells stably expressing tandem-tagged mCherry-GFP-LC3 were treated with vehicle only, 1.6 µM of compound 8.12 or with 25 nM of bafilomycin A1, for 48 h. The extent of colocalization of red and green puncta was analyzed by confocal microscopy. (D) Quantification of the area of red-only puncta in MDA-MB-231 cells expressing tandem-tagged mCherry-GFP-LC3 after treatment with vehicle only, 0.8 µM or 1.6 µM compound 8.12 was performed using ImageJ software as described in Materials and Methods. P ≤ 0.001 (***). All pictures were taken using a 63× oil immersion lens with identical optical settings throughout a single experiment. (E) PC3 cells were treated with 1.6 µM of compound 8.12, 25 nM of bafilomycin A1, or both, for 48 h, whereupon the cells were harvested and lysates were subjected to immunoblotting using an antibody against LC3. (F) HepG2 cells were transfected with siRNA targeting ATG5 (siATG5) or control siRNA and then treated with 1.2 µM compound 8.12 for 48 h. Cells were harvested and lysates subject to immunoblot analysis using an antibody to LC3. (G) The viability of HepG2 cells subjected to ATG5 knockdown was assessed with treatment using vehicle only or 2.4 µM compound 8.12 for 48 h using the MTS assay as described in Materials and Methods. P ≤ 0.05 (*). White bars: control siRNA; gray bars: siATG5. Relative cell viability was calculated as described in Figure 1. All data shown are from single experiments that have been repeated at least twice with similar results.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.