Abstract
Background
Robot-assisted laparoscopic radical prostatectomy (RALP) has become increasingly common; however, there have been no nationwide, population-based, non–claims-based studies to evaluate differences in outcomes between RALP and open radical retropubic prostatectomy (RRP).
Objective
To determine surgical, oncologic, and health-related quality of life (HRQOL) outcomes following RALP and RRP in a nationwide cohort.
Design, setting, and participants
We identified 903 men in the Health Professionals Follow-up Study diagnosed with prostate cancer between 2000 and 2010 who underwent radical prostatectomy using RALP (n = 282) or RRP (n = 621) as primary treatment.
Intervention
Radical prostatectomy.
Outcome measurements and statistical analysis
We compared patients undergoing RALP or RRP across a range of perioperative, oncologic, and HRQOL outcomes.
Results and limitations
Use of RALP increased during the study period, constituting 85.2% of study subjects in 2009, up from 4.5% in 2003. Patients undergoing RALP compared to RRP were less likely to have a lymph node dissection (51.5% vs 85.4%; p < 0.0001), had less blood loss (207.4 ml vs 852.3 ml; p < 0.0001), were less likely to receive blood transfusions (4.3% vs 30.3%; p < 0.0001), and had shorter hospital stays (1.8 d vs 2.9 d; p < 0.0001). Surgical, oncologic, and HRQOL outcomes did not differ significantly among the groups. In multivariate logistic regression models, there were no significant differences in 3- or 5-yr recurrence-free survival comparing RALP versus RRP (hazard ratios: 0.98 [95% confidence interval (CI), 0.46–2.08] and 0.75 [95% CI, 0.18–3.11], respectively).
Conclusions
In a nationwide cohort of patients undergoing surgical treatment for prostate cancer, RALP was associated with shorter hospital stay, and lower blood loss and transfusion rates than RRP. Surgical oncologic and HRQOL outcomes were similar between groups.
Patient summary
We studied men throughout the United States with prostate cancer who underwent surgical removal of the prostate. We found that robot-assisted laparoscopic radical prostatectomy resulted in shorter hospital stay, less blood loss, and fewer blood transfusions than radical retropubic prostatectomy. There were no differences in cancer control or health-related quality of life.
Keywords: Robotic, Open, Prostatectomy, Outcomes, Health-related quality of life
1. Introduction
Robotic surgery systems have disseminated rapidly throughout the United States. For prostate cancer (PCa) treatment, the proportion of prostatectomies performed robotically has risen from 8% in 2003 to 67% in 2009 [1]. This increase has taken place despite a paucity of high-quality data supporting the benefits of robot-assisted laparoscopic radical prostatectomy (RALP) over open retropubic radical prostatectomy (RRP) [2–4]. Evaluation of clinical data on perioperative outcomes of RALP and RRP are generally limited to single-institution case series in which RALP was associated with lower estimated blood loss (EBL), shorter lengths of hospital stay (LOS), lower or similar rates of positive surgical margins (PSMs), and no difference in biochemical recurrence–free survival (bRFS) [2,5,6]. The only population-based studies are restricted to claims-based data [7–9], with concerns about incomplete reporting and accuracy of data. Two of these studies were unable to differentiate between minimally invasive prostatectomy with or without the use of the robotic assistance [7,8]. In addition, studies evaluating health-related quality-of-life outcomes (HRQOL) of urinary incontinence and impotence using a validated patient-reported questionnaire among patients who had undergone RALP and those who had undergone RRP are even more sparse, with no multicenter or population studies available [2–5]. A randomized controlled study of RALP versus RRP is currently enrolling patients [10], but results will not be available for several years.
We therefore sought to evaluate surgical, oncologic, and HRQOL outcomes following RALP and RRP over a 10-yr interval in a nationwide, population-based cohort of US men with PCa.
2. Patients and methods
2.1. Study population
The men in this study are participants in the Health Professionals Follow-up Study (HPFS), a prospective study of 51 529 US male health professionals who enrolled in 1986 by completing a mailed questionnaire as previously described [11]. Participants complete biennial follow-up questionnaires to update information on new medical diagnoses and lifestyle (response rate: 96%).
After participants report a PCa diagnosis, we obtain medical records to confirm the diagnosis and record clinical information (eg, T stage, Gleason score), treatments, prostate-specific antigen (PSA) values at diagnosis, PSA levels after treatment (to identify events of biochemical recurrence), and metastasis. Participants also complete biennial follow-up questionnaires to update data on treatments, PSA levels, and clinical progression. The base population for this analysis included men who were diagnosed with PCa after January 1, 2000, and were treated with radical prostatectomy (RP) as primary therapy within 1 yr of diagnosis between 2000 and 2010. The main analysis included the 903 men treated with RALP and RRP, excluding those who had prostatectomy with a pure laparoscopic (n = 32), perineal approach (n = 28), or had unknown type (n = 102).
2.2. Surgical technique and perioperative and oncologic outcomes
The medical records of patients who underwent RALP or RRP were evaluated to determine perioperative outcomes. BRFS was defined as PSA level >0.2 ng/ml after surgery and for at least two consecutive measures (date of failure was the date of first increase) [12,13]. Men for whom we could not ascertain a PSA recurrence but who reported metastasis or died of PCa were assigned a date of recurrence as the earliest date for any of these events. We used modified D’Amico criteria as previously described (that do not distinguish between T2 substages) because the substage definitions were changed twice by the American Joint Commission on Cancer during the study period [14].
2.3. Patient-reported outcomes
We used the Expanded Prostate Cancer Index Composite 26 (EPIC-26) to assess HRQOL in the HPFS on the 2010 prostate biennial questionnaire [15,16]. Men who returned their baseline questionnaire before January 1, 2009, were eligible for this mailing, which included 650 of the 903 men who underwent RALP or RPP. The 2010 questionnaire was completed by 614 of 650 men (response rate: 94.5%). For the patient-reported outcomes analysis, we restricted the population to 600 men who completed the questionnaire ≥2 yr after prostatectomy.
We measured cancer care satisfaction using the Satisfaction Scale for Cancer Care (SCA), developed and validated by our group and previously described [15,17]. Unlike other instruments focused on satisfaction with cancer care processes, the SCA instrument is unique in providing a robust, valid measure of satisfaction with care outcome (Cronbach α = 0.88) [18]. Satisfaction data were collected on the 2010 prostate biennial questionnaire.
2.4. Statistical analysis
We compared patient and tumor characteristics, perioperative outcomes, and oncologic outcomes between RALP and RRP groups. The t test and Wilcoxon test were used to compare means and medians across groups and the Fisher exact test was used for categorical variables (p < 0.05 was considered significant). For variables that had a possible secular trend over time (ie, PSA value, biopsy Gleason score, risk score, pathologic Gleason score, and LOS), logistic or linear regression models were used to test whether there were differences by type of prostatectomy, adjusting for calendar year of surgery (continuous, years).
Recurrence was defined as any report of biochemical recurrence, metastasis, or PCa death, using the earliest date available as the recurrence date. We used a Kaplan-Meier plot to illustrate recurrence-free survival (RFS) and calculated risk of recurrence within 3 yr and 5 yr using logistic regression models adjusted for age at diagnosis, clinical stage, biopsy Gleason score, PSA at diagnosis, and calendar year of surgery.
We used linear regression models to test whether there were differences in HRQOL domains and satisfaction with cancer care outcome by type of prostatectomy, adjusting for age at diagnosis (continuous, years), PSA value at diagnosis (continuous, ng/ml), calendar year of surgery (continuous, years), and time since RP (continuous, months). All analyses were performed using SAS v.9.1 (SAS Institute Inc., Cary, NC, USA) and results with a two-sided p value <0.05 were considered statistically significant.
3. Results
3.1. Patient and tumor characteristics and use of robot-assisted laparoscopic radical prostatectomy
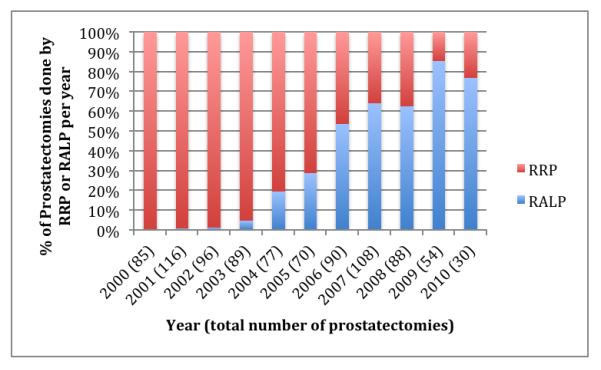
Between 2000 and 2010, 1065 men were diagnosed with PCa, of whom 282 underwent RALP and 621 underwent RRP; those in the RALP and RRP groups are the subject of this study (N = 903). Patients in the RALP group were less likely to have T2 or higher clinical stage than RRP (20.6% vs 33.0%; p = 0.0002). When corrected for year of surgery, there was no significant difference in PSA value at diagnosis (p = 0.09), biopsy Gleason score (p = 0.71), or D’Amico risk score (0.10) (Table 1). Use of RALP grew steadily over the study period, composing only 4.5% of procedures in 2003, 28.6% in 2005, 63.9% in 2007, and 85.2% in 2009 (Fig. 1). Use of RP as primary treatment in the larger cohort of men with PCa diminished slightly over time, with 40.9% of men receiving RP in 2003 and 36.5% in 2009.
Table 1.
Patient and tumor characteristics among 903 men with prostate cancer who underwent radical prostatectomy and participated in the Health Professionals Follow-up Study, 2000–2010
| Total | RALP | RRP | p value | |
|---|---|---|---|---|
| Cases, no. | 903 | 282 | 621 | |
|
| ||||
| Age at diagnosis, yr, mean | 66.0 | 67.2 | 65.4 | <0.0001 |
|
| ||||
| BMI, kg/m2, mean | 26.2 | 26.4 | 26.0 | 0.09 |
|
| ||||
| Comorbidities * | 0.45 | |||
| Yes | 17.4 | 18.8 | 16.8 | |
| No | 82.6 | 81.2 | 83.2 | |
|
| ||||
| Stage, % | 0.0002** | |||
| T1 | 70.9 | 79.4 | 67.0 | |
| T2 | 29.0 | 20.6 | 32.8 | |
| T3 | 0.1 | 0 | 0.2 | |
| T4 | 0 | 0 | 0 | |
|
| ||||
| PSA at diagnosis, ng/ml, median | 5.4 | 5.0 | 5.6 | <0.0001 |
| PSA level, ng/ml, %: | 0.09† | |||
| <4 | 16.4 | 20.7 | 14.4 | |
| 4 to <10 | 71.0 | 70.7 | 71.2 | |
| 10–20 | 9.6 | 7.9 | 10.4 | |
| >20 | 3.0 | 0.7 | 4.1 | |
|
| ||||
| Biopsy Gleason score, % | 0.71† | |||
| <6 | 2.9 | 0.7 | 3.9 | |
| 6 | 56.8 | 52.3 | 58.8 | |
| 7 | 31.4 | 37.0 | 28.9 | |
| ≥8 | 8.9 | 10.0 | 8.4 | |
|
| ||||
| Risk score§, % | 0.10† | |||
| Low | 53.5 | 48.1 | 55.9 | |
| Medium | 35.4 | 42.1 | 32.4 | |
| High | 11.1 | 9.8 | 11.7 | |
|
| ||||
| Perineural Invasion, % | 0.57 | |||
| Yes | 52.6 | 54.7 | 51.1 | |
Comorbidity status at diagnosis, considered to be yes if participant reported any of the following: myocardial infarction, coronary artery bypass or coronary angioplasty, angina confirmed by an angiogram or stress test, stroke, Parkinson disease, emphysema or chronic bronchitis, or diabetes, updated over follow-up until diagnosis.
T1 versus T2 or higher.
Adjusted for year of surgery (2000–2010).
See text for modified D’Amico definition.
Fig. 1.
Proportion of prostatectomies performed by robot-assisted laparoscopic radical prostatectomy from 2000 to 2010 in a nationwide cohort of prostate cancer patients.
RALP = robot-assisted laparoscopic radical prostatectomy; RRP = radical retropubic prostatectomy.
3.2. Perioperative outcomes
Lymph node dissection status, LOS, EBL, and transfusion status differed significantly between the treatment groups (Table 2), and these results remained significant after adjustment for calendar year of surgery. Lymph node dissection was more common in higher-risk patients than low- or intermediate-risk patients in both surgical groups (p < 0.01 for both (data not shown). However, among the low-risk patients, a higher proportion of RRP patients (77.1%) had a lymph node dissection compared to RALP patients (35.3%), as well as among intermediate-risk patients (94.8% vs 64.8%, respectively) (p < 0.0001) (data not shown). There was no difference in the proportion of nerve-sparing between RALP and RRP overall and by clinical stage (data not shown), with the majority of patients getting bilateral nerve sparing (70.7% vs 68.3%, respectively; p = 0.39), and no difference in seminal vesicle removal (95.2% vs 96.7%, respectively; p = 0.34) (Table 2).
Table 2.
Comparison of perioperative outcomes among men who underwent radical prostatectomy by robot-assisted laparoscopic radical prostatectomy versus radical retropubic prostatectomy
| Total | RALP | RRP | p value | |
|---|---|---|---|---|
| Nerve-sparing | 0.39* | |||
| Bilateral | 69.0 | 70.7 | 68.3 | |
| Unilateral | 14.9 | 15.1 | 14.8 | |
| None | 16.1 | 14.2 | 16.9 | |
|
| ||||
| Seminal vesicle removal | 0.34 | |||
| Yes | 96.3 | 95.2 | 96.7 | |
| No | 3.7 | 4.8 | 3.3 | |
|
| ||||
| Lymph node dissection, % | <0.0001 | |||
| Yes | 75.1 | 51.5 | 85.4 | |
|
| ||||
| Length of hospital stay, d | ||||
| Mean | 2.6 | 1.8 | 2.9 | <0.0001 |
| Median | 2.0 | 1.0 | 3.0 | <0.0001 |
|
| ||||
| Estimated blood loss, ml | ||||
| Mean | 654.3 | 207.4 | 852.3 | <0.0001 |
| Median | 500.0 | 150.0 | 700.0 | <0.0001 |
|
| ||||
| Transfusions, % | <0.0001 | |||
| Yes | 24.5 | 4.3 | 30.3 | |
|
| ||||
| Transfusion, units, no. | 0.16 | |||
| Mean | 2.0 | 2.7 | 2.0 | |
|
| ||||
| Prostate weight, g | 0.94 | |||
| Mean | 53.6 | 55.8 | 52.6 | |
RALP = robot-assisted laparoscopic radical prostatectomy; RRP = radical retropubic prostatectomy.
Any type of nerve sparing versus none.
Adjusted for year of surgery (2000–2010).
Patients in the RALP group compared to RRP were 82% less likely to receive a lymph node dissection (95% confidence interval [CI], 0.11–0.27; p < 0.0001). After adjusting for calendar year of surgery, RALP patients had, on average, 495 ml less EBL (95% CI, 389–601; p < 0.0001), were 83% less likely to receive a blood transfusion (95% CI, 0.07– 0.41; p < 0.0001), and experienced, on average, a 0.5-d shorter hospital stay (95% CI, 0.14–0.84; p = 0.007) (data not shown). Between 2004–2010 (when ample cases were available in both groups for comparison per year of surgery), the RRP group experienced a decrease in average successive LOS (0.18 d/yr, p = 0.001) and EBL (66 ml/yr, p = 0.01), whereas the RALP group did not (LOS: 0.11 d/yr, p = 0.10; EBL: 1.5 ml/yr, p = 0.85).
3.3. Oncologic outcomes
Overall, pathologic T stage did not differ significantly among patients who had undergone RALP and those who had undergone RRP, nor did presence of T3 or higher disease (22.2% vs 20.7%, respectively; p = 0.66), nodal metastasis (p = 0.74), Gleason score (p = 0.75, adjusted for year of surgery), or positive surgical margins (24.5% vs 23.1%, respectively; p = 0.51) (Table 3). After correcting for clinical stage, pathologic stage and PSMs remained not significantly different between the groups (data not shown).
Table 3.
Comparison of oncologic outcomes among men who underwent radical prostatectomy by robot-assisted laparoscopic radical prostatectomy versus radical retropubic prostatectomy
| Total | RALP | RRP | p value | |
|---|---|---|---|---|
| pT stage, % | 0.66* | |||
| T2 | 78.8 | 77.8 | 79.3 | |
| T3 | 21.1 | 22.2 | 20.5 | |
| T4 | 0.1 | 0 | 0.2 | |
|
| ||||
| N stage, % | 0.74** | |||
| NX | 26.5 | 48.6 | 16.6 | |
| N0 | 72.1 | 50.7 | 81.7 | |
| N1 | 1.5 | 0.7 | 1.8 | |
|
| ||||
| Pathologic Gleason score, % | 0.75† | |||
| <6 | 4.2 | 1.4 | 5.5 | |
| 6 | 38.8 | 33.6 | 41.2 | |
| 7 | 47.5 | 54.3 | 44.4 | |
| 8 | 4.0 | 3.9 | 4.0 | |
| ≥9 | 5.5 | 6.8 | 4.9 | |
|
| ||||
| Margins, % | 0.51 | |||
| Positive, multiple | 8.5 | 10.2 | 7.8 | |
| Positive, one | 15.0 | 14.3 | 15.3 | |
| Negative | 76.5 | 75.6 | 76.9 | |
|
| ||||
| Extracapsular extension, % | 0.22 | |||
| Yes | 22.8 | 20.0 | 24.0 | |
|
| ||||
| Seminal vesicle invasion, % | 0.30 | |||
| Yes | 6.5 | 5.1 | 7.2 | |
RALP = robot-assisted laparoscopic radical prostatectomy; RRP = radical retropubic prostatectomy.
T2 versus T3 or higher.
N0 versus N1.
Adjusted for year of surgery (2000–2010).
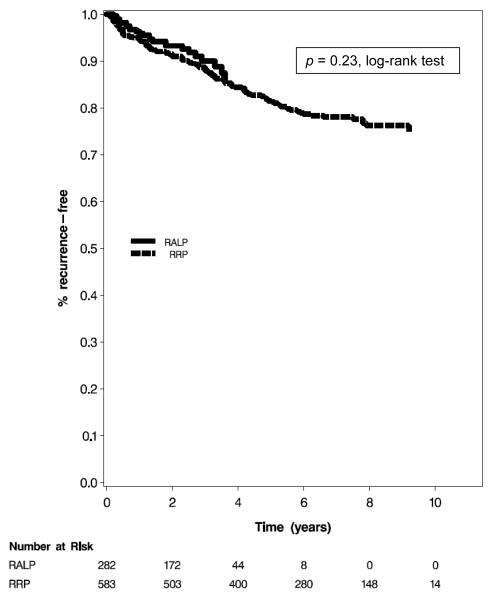
Median follow-up was 2.4 yr in the RALP group and 6.8 yr in the RRP group. There were 24 recurrence events in the RALP group (all of which were biochemical) and 116 recurrence events in the RRP group (110 of which were biochemical, 4 were metastasis, and 2 were PCa death). Kaplan-Meier analysis demonstrated no difference in RFS (p = 0.23) (Fig. 2). Median time to recurrence was 1.2 yr in the RALP group and 2.5 yr in the RRP group. Among men with ≥3 yr of follow-up (99 in the RALP group and 493 in the RRP group), 3-yr RFS was 88.9% for RALP and 89.9% for RRP. Among men with at least 5-yr of follow-up (25 in the RALP group and 393 men in the RRP group), 5-yr RFS was 88.0% for RALP and 84.7% for RRP. Logistic regression models in patients with minimum follow-up of 3 yr or 5 yr adjusted for age at diagnosis, year of surgery, clinical stage, biopsy Gleason score, and PSA value at diagnosis, demonstrated no difference in recurrence within 3 yr or 5 yr between RALP and RRP (odds ratios: 0.98 [95% CI, 0.46–2.08] and 0.75 [95% CI, 0.18–3.11], respectively).
Fig. 2.
Kaplan Meier analysis of biochemical recurrence-free survival. The analysis includes patients who are actively followed in the Health Professionals Follow-up Study for all recurrence outcomes, including biochemical recurrence, metastasis, and prostate cancer death.
RALP = robot-assisted laparoscopic radical prostatectomy; RRP = radical retropubic prostatectomy.
3.4. Health-related quality-of-life outcomes
The EPIC-26 questionnaire, which was included as part of the 2010 prostate follow-up questionnaire, was sent to 147 patients in the RALP group and 503 patients in the RRP group, with a response rate of 95.2% (140 of 147 patients) and 94.2% (474 of 503 patients), respectively. The analysis was limited to 600 men—132 patients in the RALP group and the 468 patients in the RRP group—who completed the questionnaire ≥2 yr after prostatectomy. There was no significant difference in HRQOL outcomes among the groups overall or in regard to any of the specific domains or when stratified by low- and intermediate- or high-risk groups (Table 4).
Table 4.
Comparison of patient-reported outcomes* among men who underwent radical prostatectomy by robot-assisted laparoscopic radical prostatectomy versus radical retropubic prostatectomy
| HRQOL domain** |
All RALP | All RRP |
p value† |
Low-risk RALP |
Low-risk RRP |
p value† |
Intermediate or high-risk RALP |
Intermediate or high-risk RRP |
p value† |
|---|---|---|---|---|---|---|---|---|---|
| n = 132 | n = 468 | – | n = 64 | n = 263 | – | n = 57 | n = 185 | – | |
| Response rate, % |
95.2 | 94.2 | – | 94.4 | 94.3 | – | 96.9 | 94.9 | – |
| Urinary incontinence |
74.4 ± 23.0 | 74.4 ± 25.3 | 0.93 | 69.5 ± 24.5 | 75.1 ± 26.0 | 0.42 | 81.7 ± 18.0 | 73.2 ± 24.8 | 0.12 |
| Urinary obstruction |
94.5 ± 7.5 | 93.9 ± 9.6 | 0.94 | 95.4 ± 7.6 | 93.8 ± 9.7 | 0.72 | 94.4 ± 7.4 | 93.5 ± 9.9 | 0.86 |
| Sexual | 36.8 ± 29.5 | 36.3 ± 29.7 | 0.66 | 39.4 ± 28.7 | 39.7 ± 30.0 | 0.58 | 34.2 ± 30.3 | 30.7 ± 28.1 | 0.84 |
| Bowel | 96.3 ± 9.2 | 96.3 ± 7.8 | 0.52 | 96.2 ± 10.4 | 96.3 ± 8.6 | 0.52 | 96.4 ± 8.0 | 96.1 ± 6.9 | 0.84 |
| Hormonal/vit ality |
93.5 ± 10.6 | 92.6 ± 11.4 | 0.37 | 94.2 ± 10.1 | 92.9 ± 10.8 | 0.93 | 93.2 ± 10.3 | 92.3 ± 12.1 | 0.57 |
| Outcome satisfaction |
89.3 ± 13.3 | 89.5 ± 13.6 | 0.41 | 90.5 ± 11.6 | 89.4 ± 14.3 | 0.86 | 89.9 ± 14.0 | 89.7 ± 12.7 | 0.62 |
HRQOL = health-related quality of life; RALP = robot-assisted laparoscopic radical prostatectomy; RRP = radical retropubic prostatectomy.
Up to 5 men who had RALP and up to 24 men who had RRP were missing one of the HRQOL domains from the questionnaire.
Resulting domain scores for the Expanded Prostate Cancer Index Composite 26 (urinary incontinence, urinary obstruction, sexual, bowel hormonal/vitality) is on a 0–100 scale, with higher values representing a more favorable HRQOL. Resulting scores for the Satisfaction Scale for Cancer Care (outcome satisfaction) is on a 0–100 scale, with higher values representing more satisfaction.
P values represent significance of the surgical technique variable (RALP vs RRP) in multivariable linear models, with HRQOL score as the dependent variable, adjusted for patient age at diagnosis, D’Amico risk group, and time since prostatectomy. The models by risk group were not adjusted for risk group.
4. Discussion
Despite the lack of population-based data (apart from claims data) comparing RALP to RRP, there has been rapid propagation of robotic platforms across the United States, with the majority of RPs now being performed with robotic assistance [1,19,20]. Most data come from select high-volume, single-institution series and may not be generalizable to the population has a whole [2–5]. Three population-based studies using claims-based data are limited by concerns of incomplete coding of diagnosis by urologists [7–9]. Our study provides data from a nationwide cohort of patients with centralized reporting of clinical data.
We found somewhat higher use of RALP (4.5% in 2003 to 85.2% in 2009) in comparison to a survey of urologists’ case logs reporting an increase in proportion of RALP for prostatectomy from 8% in 2003 to 67% in 2009 [1]. In addition, two studies using Medicare data found the proportion of minimally invasive prostatectomies increased from 4.9% to 9.2% in 2003 to 43.2% in 2006 and 44.5% in 2007 [7,19]. This difference may be due to our cohort being health professionals with better access to care and knowledge about new technologies with the potential for improved outcomes.
Previously reported advantages of RALP over RRP by single-institution studies were supported by our study. EBL in single-institution studies ranged from 100 ml to 300 ml for RALP and 450 ml to 800 ml for RRP; we found median values of 150 ml and 700 ml, respectively [21–23]. The 4.5-fold higher rate of blood transfusions in RRP than RALP noted in a meta-analysis [5] was supported by our findings of a 7.0-fold higher proportion of transfusions in RRP. This may be due to improvements in RALP technique over time. LOSs are consistently lower with RALP, as seen in the population-based studies using data from the Surveillance Epidemiology and End Results study and Medicare data [4,7,24,25] with median LOS of 2 d for RALP and 3 d for RRP, similar to our findings of 1 d and 3 d, respectively.
Oncologic outcomes are of paramount importance when comparing these two techniques. Surgical margins are associated with future biochemical recurrence; since no follow-up is required, many studies use surgical margins as a surrogate for oncologic efficacy. Some single-institution studies reported a lower rate of positive margins with RALP compared to RRP, ranging from 15% to 22% and from 33% to 36% [26–28], whereas others found no difference, with respective rates of 16% to 34% and 17% to 30% [5,24,25,29] similar to our findings. More important than surgical margins for evaluation of oncologic outcomes is bRFS, as this is more closely associated with mortality and dictates further cancer therapies. Only one study reported on recurrence [30] and found no difference in biochemical recurrence among men who had undergone RALP and those who had undergone RRP (16% vs 16.5%; p = 0.19), with median follow-ups of 8 mo and 17 mo, respectively. We observed no difference in RFS between groups. Because follow-up was longer in the RRP than RALP group, we performed a logistic regression analysis restricted to patients with at least 3 yr and 5 yr of follow-up, and this also showed no significant differences. Since median time to recurrence in the longer follow-up RRP group was 2.5 yr, the restriction of the subsequent recurrence-free logistic analysis to 3 yr and 5 yr would appropriately capture most events. In addition, similar to another population-based study [31], we found that patients undergoing RALP were less likely to have a lymph node dissection than the RRP group.
Another suggested benefit of RALP from early single-institution studies [32] was the potential for improved urinary incontinence and sexual function [5,32]. However, many of these studies did not use validated questionnaires and were from single institutions, potentially limiting generalizability [2]. One single-institution study using a validated questionnaire also found no difference in HRQOL [33], as we did.
Outcomes following RP, including perioperative, oncologic, and HRQOL, are multifactorial. Pretreatment patient/tumor characteristics and baseline function play major roles, as well as surgeon experience and technique. Therefore, firm conclusions cannot be drawn from any single-surgeon series. The strength of our population-based study is that all states within the United States were represented, allowing for a diverse cohort of patients treated by surgeons with varying experience and techniques. Our study does not attempt to determine whether RALP or RRP outcomes are better between the best or worst surgeon, but rather to describe outcomes within the United States.
Individual surgeons can compare their specific outcomes to national averages to determine areas where further improvements can be made.
Our study needs to be interpreted with respect to its limitations. First, although this is a nationwide cohort, it is limited to health professionals who may have different access and knowledge regarding health-care options and providers, thus influencing rates of RALP and RRP. Second, this is an observational study and factors that influenced patients to select RRP or RALP also could influence the observed outcomes; a randomized trial would be required to avoid this potential confounding. Third, our median follow-up time is limited to 2.4 yr in the RALP patients and 6.8 yr in the RRP patients. We accounted for this difference by reporting results from the Kaplan-Meier analysis and by restricting the analysis to patients with at least 3 yr or 5 yr of follow-up. Last, it is important to note that although the RRP technique was introduced more than three decades ago and has matured over time, the RALP technique was only introduced within the last decade and, therefore, continued to evolve and mature over the study period. Additional follow-up is needed to better evaluate oncologic outcomes of recurrence as well as mortality between the groups.
5. Conclusions
In this nationwide, population-based cohort study, we found that men who undergo RALP are more likely to have been diagnosed with lower clinical stage cancer and also have improved perioperative parameters including less EBL, fewer transfusions, and shorter LOS while maintaining similar HRQOL outcomes. Further follow-up is needed to evaluate more fully the oncologic outcomes of biochemical recurrence and mortality.
Acknowledgement statement
We thank the participants, Lauren McLaughlin, and other staff of the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. The authors assume full responsibility for analyses and interpretation of these data.
Funding/Support and role of the sponsor: This work was supported by grants from the US National Institutes of Health/National Cancer Institute (UM1 CA167552, T32CA009001, R25CA098566, R01CA141298) and the Prostate Cancer Foundation. The sponsors were involved in the design and conduct of the study, and collection, management, and analysis of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Mehrdad Alemozaffar had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Alemozaffar, Sanda, Stampfer, Kenfield.
Acquisition of data: Alemozaffar, Yecies, Kenfield.
Analysis and interpretation of data: Sanda, Mucci, Stampfer, Kenfield.
Drafting of the manuscript: Alemozaffar, Sanda, Yecies, Mucci, Stampfer, Kenfield.
Critical revision of the manuscript for important intellectual content: Alemozaffar, Sanda, Yecies, Mucci, Stampfer, Kenfield.
Statistical analysis: Mucci, Kenfield.
Obtaining funding: Sanda, Stampfer.
Administrative, technical, or material support: None.
Supervision: Sanda, Stampfer.
Other (specify): None.
Financial disclosures: Mehrdad Alemozaffar certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None
References
- [1].Stitzenberg KB, Wong YN, Nielsen ME, Egleston BL, Uzzo RG. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer. 2012;118:54–62. doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Duffey B, Varda B, Konety B. Quality of evidence to compare outcomes of open and robot-assisted laparoscopic prostatectomy. Curr Urol Rep. 2011;12:229–36. doi: 10.1007/s11934-011-0180-6. [DOI] [PubMed] [Google Scholar]
- [3].Kang DC, Hardee MJ, Fesperman SF, Stoffs TL, Dahm P. Low quality of evidence for robot-assisted laparoscopic prostatectomy: results of a systematic review of the published literature. Eur Urol. 2010;57:930–7. doi: 10.1016/j.eururo.2010.01.034. [DOI] [PubMed] [Google Scholar]
- [4].Lowrance WT, Tarin TV, Shariat SF. Evidence-based comparison of robotic and open radical prostatectomy. ScientificWorldJournal. 2010;10:2228–37. doi: 10.1100/tsw.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–63. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- [6].Kowalczyk KJ, Yu HY, Ulmer W, Williams SB, Hu JC. Outcomes assessment in men undergoing open retropubic radical prostatectomy, laparoscopic radical prostatectomy, and robotic-assisted radical prostatectomy. World J Urol. 2012;30:85–9. doi: 10.1007/s00345-011-0662-7. [DOI] [PubMed] [Google Scholar]
- [7].Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- [8].Lowrance WT, Elkin EB, Jacks LM, et al. Comparative effectiveness of prostate cancer surgical treatments: a population based analysis of postoperative outcomes. J Urol. 2010;183:1366–72. doi: 10.1016/j.juro.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol. 2012;61:679–85. doi: 10.1016/j.eururo.2011.12.027. [DOI] [PubMed] [Google Scholar]
- [10].Gardiner RA, Yaxley J, Coughlin G, et al. A randomised trial of robotic and open prostatectomy in men with localised prostate cancer. BMC Cancer. 2012;12:189. doi: 10.1186/1471-2407-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shappley WV, III, Kenfield SA, Kasperzyk JL, et al. Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol. 2009;27:4980–5. doi: 10.1200/JCO.2008.21.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol. 2002;20:3376–85. doi: 10.1200/JCO.2002.01.150. [DOI] [PubMed] [Google Scholar]
- [13].Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:365–9. doi: 10.1016/s0090-4295(02)02268-9. [DOI] [PubMed] [Google Scholar]
- [14].D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- [15].Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- [16].Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- [17].Kamo N, Dandapani SV, Miksad RA, et al. Evaluation of the SCA instrument for measuring patient satisfaction with cancer care administered via paper or via the Internet. Ann Oncol. 2011;22:723–9. doi: 10.1093/annonc/mdq417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shah NL, Dunn R, Greenfield TK, et al. Development and validation of a novel instrument to measure patient satisfaction in multiple dimensions of urological cancer care quality. J Urol. 2003;169(Suppl):11. [Google Scholar]
- [19].Kowalczyk KJ, Levy JM, Caplan CF, et al. Temporal national trends of minimally invasive and retropubic radical prostatectomy outcomes from 2003 to 2007: results from the 100% Medicare sample. Eur Urol. 2012;61:803–9. doi: 10.1016/j.eururo.2011.12.020. [DOI] [PubMed] [Google Scholar]
- [20].Lowrance WT, Eastham JA, Savage C, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–93. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kordan Y, Barocas DA, Altamar HO, et al. Comparison of transfusion requirements between open and robotic-assisted laparoscopic radical prostatectomy. BJU Int. 2010;106:1036–40. doi: 10.1111/j.1464-410X.2010.09233.x. [DOI] [PubMed] [Google Scholar]
- [22].Ficarra V, Novara G, Fracalanza S, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009;104:534–9. doi: 10.1111/j.1464-410X.2009.08419.x. [DOI] [PubMed] [Google Scholar]
- [23].Farnham SB, Webster TM, Herrell SD, Smith JA., Jr Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67:360–3. doi: 10.1016/j.urology.2005.08.029. [DOI] [PubMed] [Google Scholar]
- [24].Rocco B, Matei DV, Melegari S, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int. 2009;104:991–5. doi: 10.1111/j.1464-410X.2009.08532.x. [DOI] [PubMed] [Google Scholar]
- [25].Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448–53. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- [26].Di Pierro GB, Baumeister P, Stucki P, Beatrice J, Danuser H, Mattei A. A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre with a limited caseload. Eur Urol. 2011;59:1–6. doi: 10.1016/j.eururo.2010.10.026. [DOI] [PubMed] [Google Scholar]
- [27].Smith JA, Jr., Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385–9. doi: 10.1016/j.juro.2007.08.008. discussion 2389–90. [DOI] [PubMed] [Google Scholar]
- [28].White MA, De Haan AP, Stephens DD, Maatman TK, Maatman TJ. Comparative analysis of surgical margins between radical retropubic prostatectomy and RALP: are patients sacrificed during initiation of robotics program? Urology. 2009;73:567–71. doi: 10.1016/j.urology.2008.11.011. [DOI] [PubMed] [Google Scholar]
- [29].Schroeck FR, Sun L, Freedland SJ, et al. Comparison of prostate-specific antigen recurrence-free survival in a contemporary cohort of patients undergoing either radical retropubic or robot-assisted laparoscopic radical prostatectomy. BJU Int. 2008;102:28–32. doi: 10.1111/j.1464-410X.2008.07607.x. [DOI] [PubMed] [Google Scholar]
- [30].Barocas DA, Salem S, Kordan Y, et al. Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival. J Urol. 2010;183:990–6. doi: 10.1016/j.juro.2009.11.017. [DOI] [PubMed] [Google Scholar]
- [31].Prasad SM, Keating NL, Wang Q, et al. Variations in surgeon volume and use of pelvic lymph node dissection with open and minimally invasive radical prostatectomy. Urology. 2008;72:647–52. doi: 10.1016/j.urology.2008.03.067. discussion 652–3. [DOI] [PubMed] [Google Scholar]
- [32].Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205–10. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- [33].Malcolm JB, Fabrizio MD, Barone BB, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol. 2010;183:1822–8. doi: 10.1016/j.juro.2009.12.102. [DOI] [PubMed] [Google Scholar]