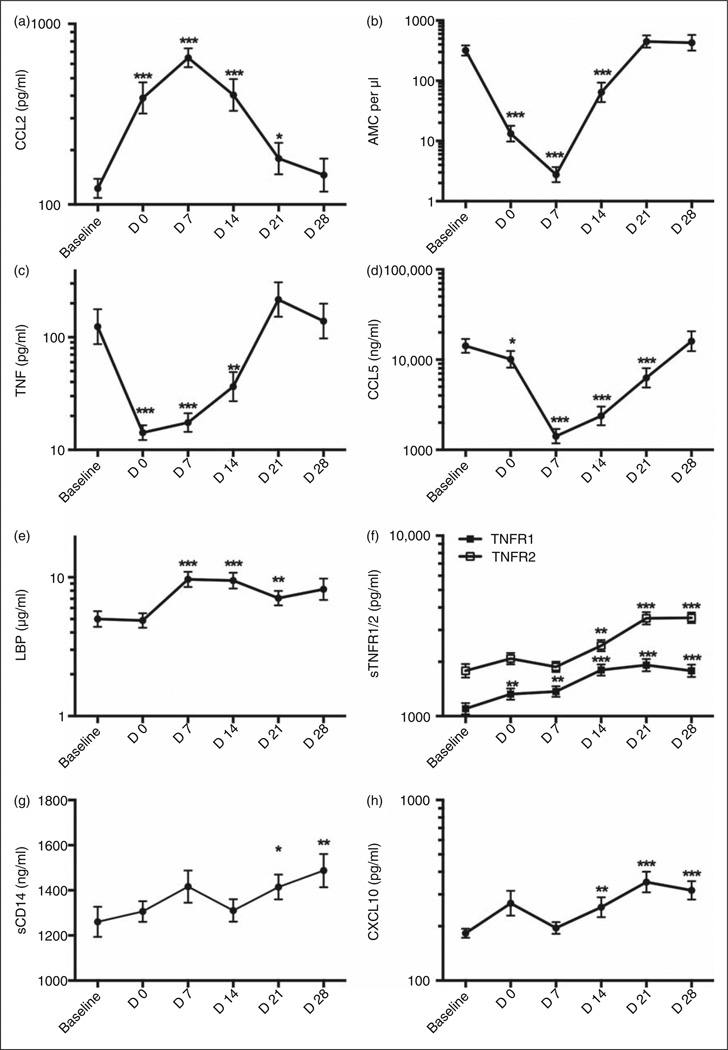
Figure 1.
In vivo and ex vivo cellular and plasma changes in inflammatory responses following onset of endotoxemia in myeloablative HCT. Cohort I: peripheral blood and plasma samples from patients undergoing HCT were collected at baseline (B—before myeloablative conditioning), d 0 (day of transplant) and subsequent d 7, 14, 21 and 28, and analyzed for (a) CCL2 protein (pg/ml), (b) absolute monocyte count (AMC) per µl, (c) spontaneously produced TNF-a protein (pg/ml), (d) CCL5 protein (ng/ml), (e) LBP protein (µg/ml), (f) TNF-α receptor 1 and 2 (TNFR1/2) protein (pg/ml), (g) soluble CD14 (sCD14) protein (ng/ml) and (h) CXCL10 protein (pg/ml) over time. Values are shown as mean ± SEM for n = 22–48 patients. Statistical significance compared to B was assessed using Wilcoxon signed rank test for matched pairs and is indicated as *P < 0.05 **P < 0.01 and ***P<0.001.