Abstract
Plasmablastic lymphoma (PBL) is an uncommon malignancy which predominantly occurs in the oral cavity of human immunodeficiency virus (HIV)-positive patients. Sporadic cases have been published describing PBL in immunocompetent patients as well as in immunodeficient patients following immunosuppressive therapy or transplantation. We hereby reported a case of PBL in a 69-year-old, HIV-negative male subjected to combination treatment with fludarabine and rituximab for nongastric mucosa-associated lymphoid tissue (MALT) lymphoma. The diagnosis of PBL was made with tumor cells of immunoblasts or plasmablasts morphology strongly positive for MUM-1, EMA and CD138, and partly positive for CD38, and negative for CD20, BCL-6, and CD56, and approximately 80% of which were positive for Ki-67. The case presented PBL after MALT, and a history of chemotherapy including fludarabine and rituximab led to the potential immunocompromised state. The patient died 5 months after the diagnosis of PBL.
Keywords: Plasmablastic lymphoma, non-gastric mucosa-associated lymphoid tissue lymphoma, case report
Introduction
PBL was characterized by a diffuse proliferation of large neoplastic cells resembling immunoblasts or plasmacytes with the almost identical immunophenotype of plasma cells [1]. It was first reported occurring in the oral cavity in the setting of HIV by Delecluse et al [2], originally described as a distinct subtype of diffuse large B-cell lymphoma (DLBCL). Most cases were observed in HIV-positive patients with a predilection to EBV infection and oral cavity. Over the years, various cases have been reported in the absence of HIV, particularly in patients with immunosuppression after malignancies such as indolent B-cell lymphomas, and solid organ or bone marrow transplantations [3-9] in immunocompetent patients as well [10,11]. PBL involving in the extraoral sites has been observed but not common while oral cavity involvement is the most frequent [12]. We here present a case of PBL with cytogenetic abnormality was diagnosed after immunosuppressive chemotherapy including fludarabine and rituximab for marginal zone lymphoma (MZL) two and a half years later.
Case report
A 69-year-old male with a past medical history of hypertension was admitted to our hospital in September 2008 for a three-month history of bilateral slow-growing submandibular masses. There was no superficial lymph node palpable and no hepatosplenomegaly. He had a resection of the left submandibular gland and the pathology revealed MALT lymphoma. CT scans showed retroperitoneal generalized lymphadenopathy, favoring lymphoma. No morphologic evidence of lymphoma was noted in the bone marrow aspiration and trephine biopsy. HIV, EBV-DNA and hepatitis serologies were negative.
The patient was diagnosed with stage IIIA nongastric MALT lymphoma, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0, and was treated with 3 cycles of rituximab (375 mg/m2 on day 1), but a new CT scan following the treatment revealed almost no change in the number or size of lesions reported before. The patient subsequently received 6 cycles of FC (fludarabine 25 mg/mm2 and cyclophosphamide 250 mg/mm2 on days 1 to 3), and after four cycles of FC, CT showed complete remission. He completed therapy and was then observed under surveillance of blood tests and CT scans regularly.
He remained stable; however, two and a half years later, the patient presented with lower abdominal distension and had a 10 cm × 4 cm left cystic inguinal mass which was noted as left funicular hydrocele on B ultrasonic examination. He underwent an operation of the lesion, and the pathology was hyperplasia of fibrous tissue with inflammation. The patient suffered from slight left lower abdominal distension and constipation after the operation but rejected to be further treated. However, he sought care after four months because of intermittent hematuria and night sweats. A 10 cm × 9 cm left abdominal mass was palpable and CT scan finding showed mass lesions in the posterior bladder wall and spermatic cord area, and swollen lymph nodes in the left perirenal region, retroperitoneum and pelvic cavity, suggesting tumor infiltration. A CT-guided percutaneous puncture biopsy of the left retroperitoneal mass was performed and the pathology revealed fibrous tissue involvement of tumor cells, favoring lymphoma relapse. Histologic evaluation confirmed the presence of large neoplastic cells morphologically resembling immunoblasts or plasmablasts. On immunohistochemistry, the neoplastic cells were strongly positive for MUM-1, EMA and CD138, and partly positive for CD38, and negative for CD3, CD20, CD43, CD2, BCL-6, ALK, CD56, and CD30. The Ki-67 proliferation index was 80% and these cells showed lambda light chain restriction (Figure 1). Bone marrow aspirate smears were predominantly composed of lymphoblasts and prolymphocytes (21.2% of all nucleated cells) (Figure 2) while the bone marrow trephine biopsy findings included a normal hematopoiesis and a small amount of lymphoblasts and prolymphocytes scattered amidst bone trabecular, suspecting lymphocytes involvement (Figure 3). Therefore, the patient was diagnosed of PBL with stage IVB, an ECOG performance status of 1, and International Prognostic Index (IPI) of 3, indicating poor prognosis. At the time of diagnosis, he had WBC of 9.37 × 109/L, hemoglobin (Hb) of 111 g/L, platelet count of 201 × 109/L, lactate dehydrogenase (LDH) of 1961 U/L, and ferritin of 1080 μg/L. His routine urine test revealed urine protein++, urine latent blood+. The presence of IgH gene rearrangements was indicated by PCR. Cells from bone marrow were performed chromosome banding analysis after 24h culture with the result of 51,XY,+7,+7,der(8)t(7;8)(p14;p21),+12,+15,+19 [5]/51,XY,+7,+7, der(8)t(7;8)(p14;p21),+12,+15,+der(19)t(1;19)(q23;p13) [5].
Figure 1.
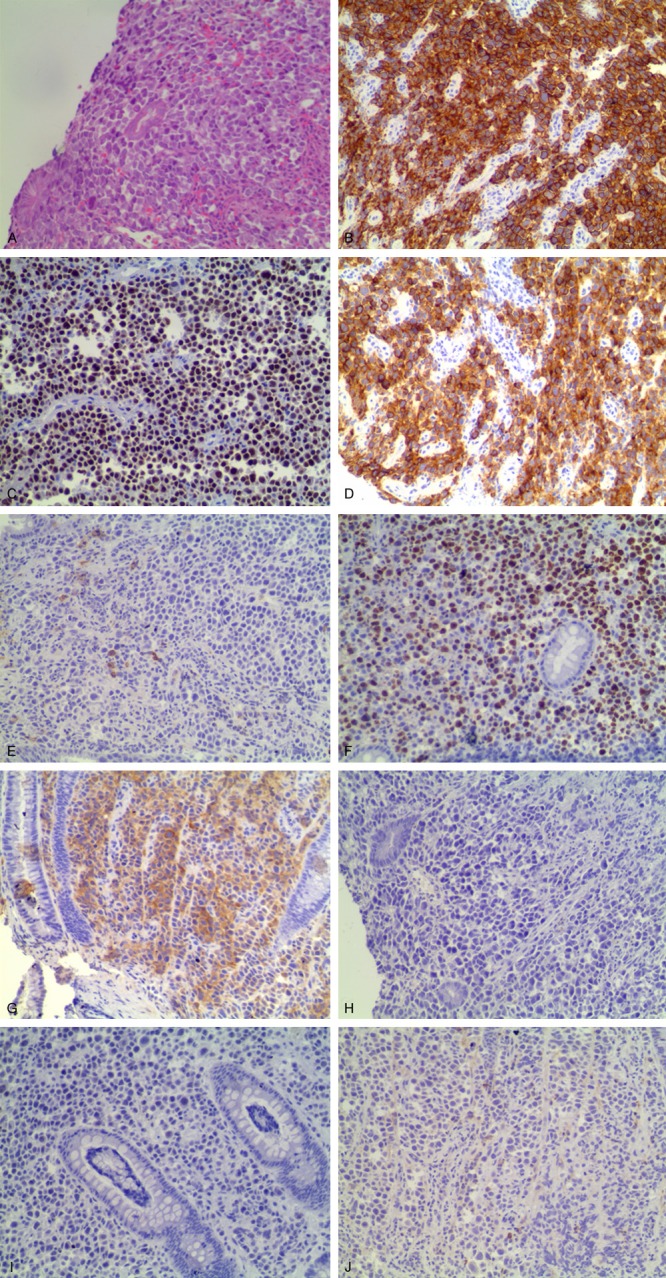
Immunophenotypic findings of plasmablastic lymphoma. A: The H&E stained section a diffuse proliferation of large atypical lymphoid cells (× 200). B-E: Immunohistochemical staining showed that the tumor cells strongly expressed CD138 (B: × 200), MUM1 (C: × 200), and EMA (D: × 200), partly expressed CD38 (E: × 200). F: Ki-67 staining was approximately 80% (× 200). G: These cells were positive for lambda light chain (× 200). H: Staining for Epstein-Barr virus (EBV)-encoded RNA by in situ hybridization (EBV EBER-ISH) showed negative nuclear staining (× 200). I, J: The tumor cells were negative for CD20 (I: × 200) and CD56 (J: × 200).
Figure 2.
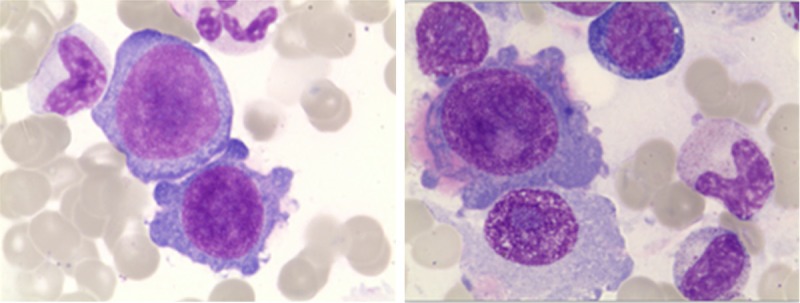
The bone marrow biopsy showing lymphoblasts and prolymphocytes consisted of 21.2% of all nucleated cells (H&E, × 400).
Figure 3.
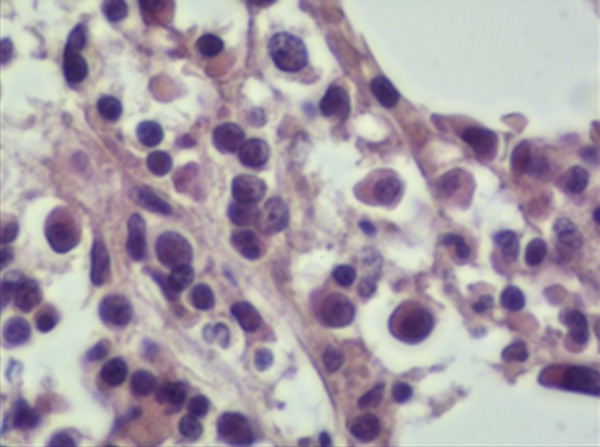
The bone marrow biopsy showing a normal hematopoiesis and a small amount of lymphoblasts and prolymphocytes scattered amidst bone trabecular. These were medium-sized cells with medium cytoplasm, and the nuclei were round or irregular partly contained nucleoli (H&E, × 400).
The patient was subjected to DA-EPOCH (doxorubicin 10 mg/m2, vincristine 0.5 mg, and etoposide 50 mg/m2 on days 1-4, prednisone 60 mg/m2 on days 1-5, CTX 750 mg/m2 on day 5). Despite he had a good response with obvious remission of abdominal distension and a substantial decrease in the size of left abdominal mass, after the second cycle of DA-EPOCH, the size of left abdominal mass increased and abdominal distension was presented. Repeat PET/CT scans revealed tumor progression. In January 2013, the patient began a second line chemotherapy with bortezomib 1.3 mg/m2 on days 2 and 5, and MINE (mitoxantrone 8 mg/m2 on day 1, ifosfamide 1333 mg/m2 and etoposide 65 mg/m2 on days 1-3).
In early February 2013, he developed left abdominal pain, night sweats and no feces for nearly a week after two cycles of regimen. The size of left abdominal mass palpably increased. His WBC was 4.52 × 109/L; Hb, 99 g/L; platelet count, 134 × 109/L; LDH, 675 U/L; ferritin, 822.5 ug/L; urine protein, +-; urine latent blood, -; red blood cells, 8 Ery/μL. Considering the patient’s age, he was subsequently treated with gemcitabine 1 g/m2 and oxaliplatin 100 mg/m2 on day 1. Unfortunately, the patient’s clinical manifestation deteriorated, with the onset of severe edema of bilateral lower extremity and scrotum. CT scans showed generalized increased swollen lymph nodes (Figure 4). The patient rejected local radiotherapy as palliative therapy and died of cardiorespiratory failure 1 month later. The tests for HIV and EBV-DNA, which were performed at every admission, were negative.
Figure 4.
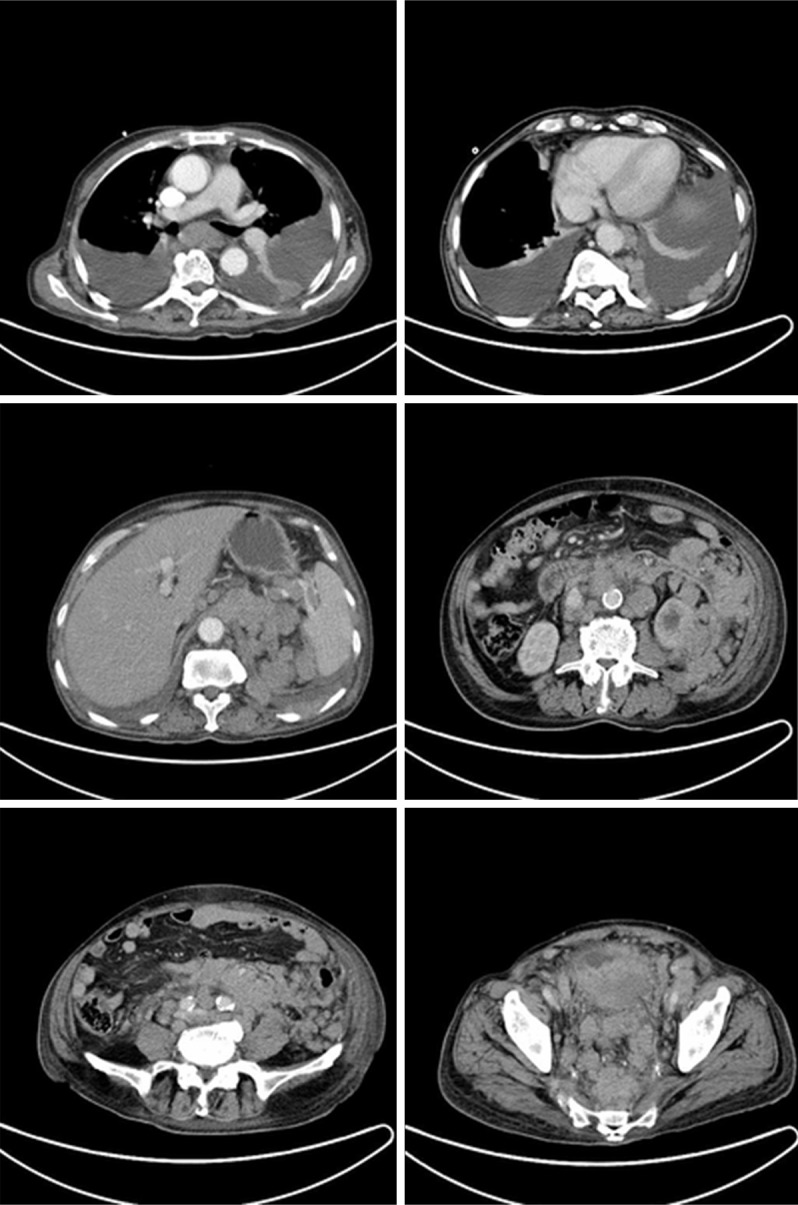
Computed tomography (CT) of abdomen. CT scans showed generalized increased swollen lymph nodes and an increase of bilateral pleural effusion. The boundary between mass and bladder was not clearly demarcated with connective tissue and a structural abnormality of the pelvis was revealed.
Discussion
PBL is a rare neoplasm of non-Hodgkin B cell lymphoma, with a morphological spectrum varying from cells resembling immunoblasts to cells with plasmacytic differentiation Historically, the median age at presentation of HIV-infected patients was 38 years and 57 years of HIV-negative patients. The male predominance persisted among both HIV-positive and negative patients [13,14]. An extensive literature review by Castillo et al [15] showed of the 228 patients of PBL, 69% were HIV-positive and 31% were HIV-negative. About one-third of HIV-negative patients presented with underlying immunosuppressive state [14]. Meanwhile, sporadic cases of immunocompetence have been reported [10,11]. Seemingly, there is a strong connection between EBV infection and PBL, which has been reported that EBV infection was detected in 72% of PBL cases [15]. The association between HHV-8 and PB is still elusive with rare cases reported [16-18]. In our report, the patient was negative for HIV and EBV DNA serologically; however, the history of MZL and chemotherapy including fludarabine and rituximab probably led to the immunocompromised state.
Previous clinical studies have established that HIV-positive PBL presents most frequently in the oral cavity, and extraoral sites are becoming increasingly recognized among which the common sites of presentation included the gastrointestinal tract and lymph nodes [13]. With the most common sites being the oral cavity, the gastrointestinal tract and soft tissue, 89% of the cases of HIV-negative PBL described at least one extranodal site involved [12].
With regards to immunophenotype, the typical surface marker of PBL is positive for at least one plasmacytic marker such as CD138, or MUM-1, with a negative or weak expression of mature B-cell surface marker such as CD20, CD19 and PAX5. In approximately 50%-85% of the cases, CD79a is positive [1]. By means of in situ hybridization (ISH), the presence of EBV in HIV-associated and HIV-negative PBL were 82% and 46%, respectively. The expression of Ki-67 at ≥ 80% was seen in over 70% patients of PBL [15]. HIV-negative individuals have a much lower expression of CD56 [15]. Positive regulatory domain 1 (PRDM1/BLIMP1) and X-box binding protein 1 (XBP1), which are associated with terminal differentiation program, are frequently expressed [19,20]. T-cell surface markers CD3 and CD4 are rarely expressed [15,21].
The morphological differential diagnosis in-cludes a spectrum of B-cell lymphomas with plasmablastic features such as DLBCL, primary effusion lymphoma (PEL), plasma cell myeloma or plasmacytoma, and HHV-8-associated multicentric Castleman disease lymphoma [20]. These differential diagnoses are supported by their consistency with immunophenotype of tumor cells and clinical manifestation. Plasmablastic phenotype with positive PRDM1/BLIMP1 and XBP1, strong reactivity of CD138 and CD38, and negative or weakly positive CD20/PAX5 helps to differentiate PBL from DLBCL [19]. PEL is universally associated with HHV8 and co-infected with EBV [1]. Clinical features such as the presence of bone lesions and serum monoclonal proteins can greatly contribute to the diagnostic differentiation between plasmacytoma and PBL [20].
Valera et al [22] identified MYC rearrangements in 20 cases (49%) using fluorescence in situ hybridization (FISH), and additionally found gains in three or more loci in 30% of the PBL. A case of follicular lymphoma (FL) that transformed to PBL was demonstrated by identifying maintain the IGH/BCL2 translocation and acquired MYC rearrangement based on analysis of PCR and FISH [5]. The patient we reported here harbored complex karyotype with a gain of five loci at the time of being diagnosed with PBL by chromosome banding analysis. We failed to get his initial chromosomal condition and immunophenotype despite our efforts to gather data. Thus, the connection between his diagnosis of MZL and PBL was not fully understood. It was possible that PBL occurred as a secondary malignancy in the context of potential immunosuppression after chemotherapy for MZL. Recently, Martinez et al [6] reported 3 cases of chronic lymphocytic lymphomas (CLL) and 3 cases of FL that had a PBL-transformation at the initial diagnosis or in the evolution of the disease by showing similar plasmablastic morphologic and phenotypic features. Given the fact that low-grade B-cell lymphoma may transform into a more aggressive lymphoma, possibility that a plasmablastic transformation of MZL cannot be excluded.
A review of 70 HIV-positive patients receiving chemotherapy showed the overall response rate (ORR) was 77% [23], while in 42 HIV-negative patients at least 69% achieved a PR [14]; however, despite a good response to chemotherapy, the median overall survival (OS) for the entire group was 12 months with an estimated 5-year OS of 21% in 138 patients with PBL [15]. The large-scale prospective cohort study on treatment for PBL has not been carried out attribute to the scarcity of cases. The current National Comprehensive Cancer Network (NCCN) guidelines recommend intensive chemotherapy such as CODOX-M/IVAC (modified), Dose-adjusted EPOCH, and HyperCVAD [24], whereas a study showed there was no statistical difference comparing 35 patients of PBL receiving CHOP or CHOP-like chemotherapy and 16 patients treated with more intensive regimens leaving the role of more intensive regimens is still obscure [23]. Nonetheless, more intensive therapeutic approaches are needed in patients with poor prognosis. Chemotherapy with bortezomib was proved to be of value in some cases [25,26], though not helpful in our case. The loss of CD20 in PBL contributes to the useless of rituximab, but it might be beneficial in some atypical cases expressing CD20 with CD4+ cell counts over 100/mm3 taken into consideration [23]. Meta-analysis result showed that highly active anti-retroviral therapy (HAART) in addition to chemotherapy and/or radiotherapy was effective in improving the prognosis of HIV-positive PBL [27]. Four patients receiving autologous hematopoietic stem cell transplant (HSCT) after CR1 had a median survival of 27.5 months [14]. Further studies between multicenters are necessary to assess the efficiency of regimens given the scant cases of PBL and aggressive clinical course.
To date, several factors were associated with poor prognosis, including age > 60, Ann Arbor III or IV, ECOG ≥ 2, primary lesion in oral cavity, immunosuppression, bone marrow involvement, EBV infection, MYC rearrangement, CD4+ cell counts < 0.2 × 109/L and lymphocytes counts < 1.0 × 109/L [14,23,28].
In conclusion, we present a case of PBL arising from a history of combination treatment with fludarabine and rituximab for MZL. We recognize that the absence of initial karyotype result in the vague relation between MZL and PBL. To our knowledge, it is the first report of PBL after MZL.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 30971296, 81170488, 81370657), the Natural Science Foundation of Jiangsu Province (Grant No. BK2010584), Key Projects of Health Department of Jiangsu Province (Grant No. K201108), Jiangsu Province’s Medical Elite Program (Grant No. RC2011169), National Public Health Grand Research Foundation (Grant No. 201202017), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institute (Grant No. JX10231801), the Program for Development of Innovative Research Teams in the First Affiliated Hospital of Nanjing Medical University, and Project of National Key Clinical Specialty.
Disclosure of conflict of interest
None.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: France IARC Press; 2008. [Google Scholar]
- 2.Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, Huhn D, Schmidt-Westhausen A, Reichart PA, Gross U, Stein H. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413–1420. [PubMed] [Google Scholar]
- 3.Clark SW, Taylor J, Wang DL, Abramson JS, Batchelor TT. Plasmablastic lymphoma after standard-dose temozolomide for newly diagnosed glioblastoma. Neurology. 2013;81:93–194. doi: 10.1212/WNL.0b013e318297eea6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redmond M, Quinn J, Murphy P, Patchett S, Leader M. Plasmablastic lymphoma presenting as a paravertebral mass in a patient with Crohn’s disease after immunosuppressive therapy. J Clin Pathol. 2007;60:80–81. doi: 10.1136/jcp.2006.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouansafi I, He B, Fraser C, Nie K, Mathew S, Bhanji R, Hoda R, Arabadjief M, Knowles D, Cerutti A, Orazi A, Tam W. Transformation of follicular lymphoma to plasmablastic lymphoma with c-myc gene rearrangement. Am J Clin Pathol. 2010;134:972–981. doi: 10.1309/AJCPWY1SGJ9IEAOR. [DOI] [PubMed] [Google Scholar]
- 6.Martinez D, Valera A, Perez NS, Sua Villegas LF, Gonzalez-Farre B, Sole C, Gine E, Lopez-Guillermo A, Roue G, Martinez S, Sant F, Warzocha K, Robak T, Czader M, Villamor N, Colomo L, Campo E, Martinez A. Plasmablastic transformation of low-grade B-cell lymphomas: report on 6 cases. Am J Surg Pathol. 2013;37:272–281. doi: 10.1097/PAS.0b013e31826cb1d1. [DOI] [PubMed] [Google Scholar]
- 7.Borenstein J, Pezzella F, Gatter KC. Plasmablastic lymphomas may occur as post-transplant lymphoproliferative disorders. Histopathology. 2007;51:774–777. doi: 10.1111/j.1365-2559.2007.02870.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Vrancken MJ, Keglovits L, Krause J. Plasmablastic lymphoma following transplantation. Proc (Bayl Univ Med Cent) 2013;26:152–1555. doi: 10.1080/08998280.2013.11928941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen DB, Song QJ, Chen YX, Chen YH, Shen DH. Clinicopathologic spectrum and EBV status of post-transplant lymphoproliferative disorders after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2013;97:117–124. doi: 10.1007/s12185-012-1244-1. [DOI] [PubMed] [Google Scholar]
- 10.Rao DD, Aggarwal N, Anehosur V, Doddihal H, Shiraganvi M, Gopalkrishnan K. Plasmablastic lymphoma of the oral cavity in immunocompetent patients: report of two cases. Int J Oral Maxillofac Surg. 2010;39:1036–1039. doi: 10.1016/j.ijom.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi M, Ogawa F, Onishi T, Moriyama Y. Plasmablastic lymphoma in an elderly immunocompetent patient. Pathol Int. 2012;62:347–350. doi: 10.1111/j.1440-1827.2012.02798.x. [DOI] [PubMed] [Google Scholar]
- 12.Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Colvin GA, Butera JN. HIV-negative plasmablastic lymphoma: not in the mouth. Clin Lymphoma Myeloma Leuk. 2011;11:185–189. doi: 10.1016/j.clml.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804–809. doi: 10.1002/ajh.21250. [DOI] [PubMed] [Google Scholar]
- 14.Liu JJ, Zhang L, Ayala E, Field T, Ochoa-Bayona JL, Perez L, Bello CM, Chervenick PA, Bruno S, Cultrera JL, Baz RC, Kharfan-Dabaja MA, Raychaudhuri J, Sotomayor EM, Sokol L. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: a single institutional experience and literature review. Leuk Res. 2011;35:1571–1577. doi: 10.1016/j.leukres.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Colvin G, Butera JN. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma. 2010;51:2047–2053. doi: 10.3109/10428194.2010.516040. [DOI] [PubMed] [Google Scholar]
- 16.Cioc AM, Allen C, Kalmar JR, Suster S, Baiocchi R, Nuovo GJ. Oral plasmablastic lymphomas in AIDS patients are associated with human herpesvirus 8. Am J Surg Pathol. 2004;28:41–46. doi: 10.1097/00000478-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Verma S, Nuovo GJ, Porcu P, Baiocchi RA, Crowson AN, Magro CM. Epstein-Barr virus- and human herpesvirus 8-associated primary cutaneous plasmablastic lymphoma in the setting of renal transplantation. J Cutan Pathol. 2005;32:35–39. doi: 10.1111/j.0303-6987.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 18.Ustun C, Reid-Nicholson M, Nayak-Kapoor A, Jones-Crawford J, McDonald K, Jillella AP, Ramalingam P. CNS involvement, coexistence of other malignancies, possible viral etiology, and dismal outcome. Ann Hematol. 2009;88:351–358. doi: 10.1007/s00277-008-0601-x. [DOI] [PubMed] [Google Scholar]
- 19.Montes-Moreno S, Gonzalez-Medina AR, Rodriguez-Pinilla SM, Maestre L, Sanchez-Verde L, Roncador G, Mollejo M, García JF, Menarguez J, Montalbán C, Ruiz-Marcellan MC, Conde E, Piris MA. Aggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype. Haematologica. 2010;95:1342–1349. doi: 10.3324/haematol.2009.016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsi ED, Lorsbach RB, Fend F, Dogan A. Plasmablastic lymphoma and related disorders. Am J Clin Pathol. 2011;136:183–194. doi: 10.1309/AJCPV1I2QWKZKNJH. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Yoshida T, Nakamura N, Kamata H, Kotani S, Ohsaka M, Kajita S, Miyazaki K, Ohtani S, Nakayama M, Horie R, Hayakawa K, Niitsu N, Higashihara M. CD3- and CD4-positive plasmablastic lymphoma: a literature review of Japanese plasmablastic lymphoma cases. Intern Med. 2010;49:1801–1805. doi: 10.2169/internalmedicine.49.3164. [DOI] [PubMed] [Google Scholar]
- 22.Valera A, Balagué O, Colomo L, Martínez A, Delabie J, Taddesse-Heath L, Jaffe ES, Campo E. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol. 2010;34:1686–1694. doi: 10.1097/PAS.0b013e3181f3e29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Colvin G, Butera JN. Prognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphoma. Oncologist. 2010;15:293–199. doi: 10.1634/theoncologist.2009-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCCN Practice Guidelines in Oncology. AIDS-related B-cell lymphomas (AIDS-3) 2013 Available at http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. [Google Scholar]
- 25.Bibas M, Grisetti S, Alba L, Picchi G, Del Nonno F, Antinori A. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J. Clin. Oncol. 2010;28:e704–e708. doi: 10.1200/JCO.2010.30.0038. [DOI] [PubMed] [Google Scholar]
- 26.Bose P, Thompson C, Gandhi D, Ghabach B, Ozer H. AIDS-related plasmablastic lymphoma with dramatic, early response to bortezomib. Eur J Haematol. 2009;82:490–492. doi: 10.1111/j.1600-0609.2009.01235.x. [DOI] [PubMed] [Google Scholar]
- 27.Guan B, Zhang X, Ma H, Zhou H, Zhou X. A meta-analysis of highly active anti-retroviral therapy for treatment of plasmablastic lymphoma. Hematol Oncol Stem Cell Ther. 2010;3:7–12. doi: 10.1016/s1658-3876(10)50050-5. [DOI] [PubMed] [Google Scholar]
- 28.Castillo JJ, Furman M, Beltrán BE, Bibas M, Bower M, Chen W, Díez-Martín JL, Liu JJ, Miranda RN, Montoto S, Nanaji NM, Navarro JT, Seegmiller AC, Vose JM. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012;118:5270–5277. doi: 10.1002/cncr.27551. [DOI] [PubMed] [Google Scholar]


