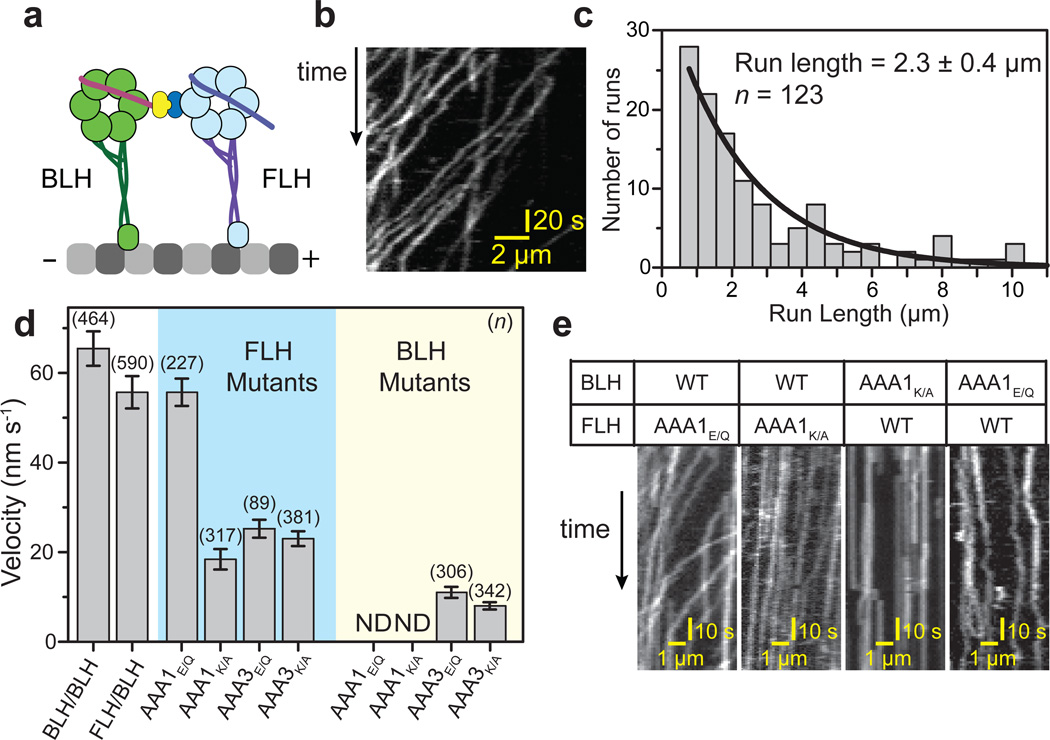
Figure 3. The linker provides force to drive the motility of a dynein dimer.
(a) Dimerization of the C-terminal ring of one head to the N-terminal tail of the other results in a dimer of a free-linker head (FLH) and a bound-linker head (BLH). (b) Kymograph showing that the FLH/BLH heterodimer is capable of processive motility. (c) Run length histogram of FLH/BLH at 2 mM ATP, with maximum likelihood fit (± 95% CI). (d) The average velocities (± SEM) of FLH/BLH constructs carrying an ATPase mutation in either the AAA1 or AAA3 site in one head (ND: motility not detected). (e) Kymographs of ATPase mutants of the FLH or BLH at 2 mM ATP. The AAA1K/A mutation on BLH abolishes directional motility, whereas AAA1E/Q mutation leads to non-directional diffusion along the MT. The same mutations on FLH do not stop motility, indicating that BLH monomer is responsible for FLH/BLH motility.