Figure 1.
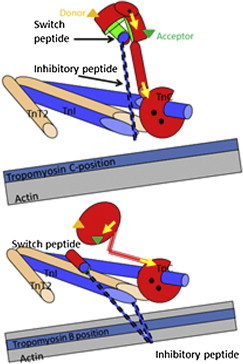
The thin-filament calcium switch. The Tn complex in the thin filament based on crystal structures of skeletal muscle TnC (red) with TnI (blue), and TnT2 (brown). This shows the role of the switch and inhibitory peptides of TnI in transmitting the calcium binding to TnC to the tropomyosin (pale blue strand) position on actin filament (gray strand). (Upper panel) Calcium-bound state. Calcium (black dots) binding to the N-terminal domain causes the regulatory domain to open, allowing the TnI signal peptide to bind in the cleft (the TnC and switch-peptide contact surface is shown in green). This inhibits the peptide from binding to its site on actin. Tm sits in its preferred C- or calcium-induced site on actin. (Lower panel) Calcium-free state with the inhibitory TnI peptide binding to actin and locating Tm in the Blocked position on actin. Note the closed regulatory domain of TnC. Note also that the diagram shows the role of the inhibitory peptide but not the rest of the C-terminus of TnI, which also contributes to the actin binding site. This is based on the crystal structure of skeletal muscle troponin core domain. The crystal structure of the cardiac troponin core domain does not show the central helix straightening as depicted here. This region may therefore be quite mobile. The locations of the fluorescent labels are for illustration only: (green and orange triangles) the FRET probes in the N-domain of TnC; (yellow arrows) the two bifunctional orientation probes in the two TnC domains. The figure is based on an original drawing by M. Vinogradova and R. Fletterick (3). To see this figure in color, go online.
