Figure 5.
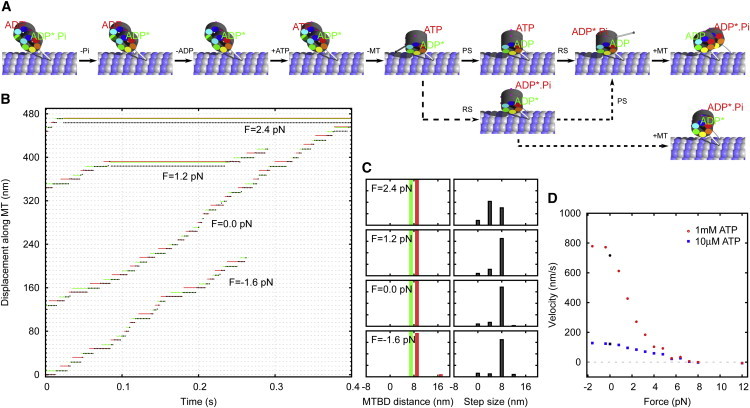
Single-molecule kinetics of tightly coupled dimers. (A) The most probable kinetic pathways for a dimeric dynein molecule. The main path (continuous arrows) leads to an advancing step. If the binding of one head occurs before the power stroke in its partner head, this can lead to a futile step (dashed arrows). (B) A portion of the on-axis stepping trace of a dimeric dynein molecule for different values of the load. Trace of the tail of the dynein (black) and the corresponding x-coordinates of the MTBD of the left (red) and the right (green) head are depicted. (C) Binding site distance and step size (measured at the tail) distributions for the respective loads. (Red and green bars) Steps taken by the left and right heads, respectively. (D) Force velocity diagram for two ATP concentrations, [ATP] = 1 mM and [ATP] = 10 μM. To see this figure in color, go online.
