Figure 1.
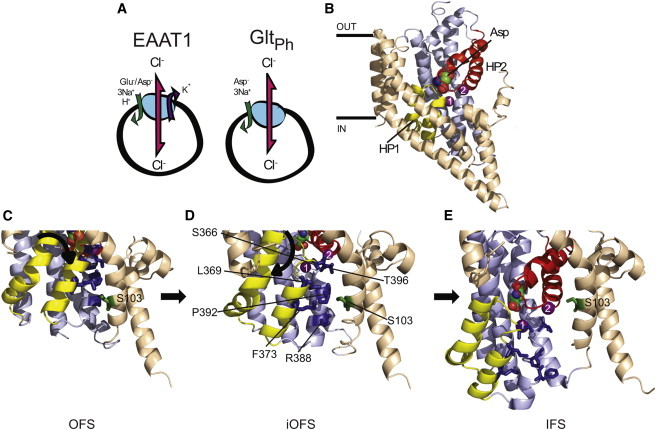
Transport stoichiometry of EAAT1 and GltPh and the putative glutamate transporter chloride channel. (A) Stoichiometry of ion-flux coupling for EAAT1 and GltPh. (B) The structure of a single protomer of GltPh in the outward-facing state (OFS; PDB:2NWX). (Space-filling representation) Bound aspartate; (purple spheres) two Na+ ions (the third Na+ ion is not shown). (Light brown) Trimerization domain; (light blue) transport domain; (yellow) HP1; (red) HP2. (C–E) Close-up of the region surrounding Ser-103 (Ser-65 in GltPh; shown in green) in the OFS (PDB:2NWX), the intermediate outward-facing state (iOFS; PDB:3V8G), and the IFS (PDB:3KBC). Residues investigated in this study are highlighted (dark blue) and labeled in panel D; TM5 has been removed for visual clarity. Figure was made using the software PYMOL (31).
