Abstract
BACKGROUND:
This study aimed to determine the plasma levels of urokinase-type plasminogen activator (uPA), urokinase-type plasminogen activator receptor (uPAR), D-dimer, IL-6 and TNF-α and observe the relations among uPA, uPAR, D-dimer, IL-6 and TNF-α in patients with systemic inflammatory response syndrome (SIRS).
METHODS:
A prospective, clinical case-control study was conducted in patients with SIRS at age of more than 55 years old treated during 2008-2010 at Wuhan Central Hospital. Venous blood samples were collected by routine venipuncture. Eighty-five patients were divided into two groups according to diagnostic criteria of SIRS: SIRS patients from intensive care units (n=50), and non-SIRS patients from medical wards (n=35). Thirty healthy blood donors who visited the General Health Check-up Division at Wuhan Central Hospital served as controls. Excluded from the study were (1) those patients with pregnancy; (2) those with cancer; (3) those died after admission into the ICU in 7 days; (4) those received cardiopulmonary resuscitation; (5) those who had previous blood system diseases; and (6) those with SIRS before admission into the ICU. The levels of uPA, uPAR, D-D, IL-6 and TNF-α in blood were detected by commercial enzyme-linked immunosorbent assay (ELISA) kit. The data were analyzed using SPSS version 17.0 and expressed as mean ± standard. Student's t test and the Mann-Whitney U test were used in the analysis. The relations of uPA, uPAR and D-dimer, IL-6 TNF-α levels were analyzed using Spearman's rank-order correlation coefficient test.
RESULTS:
The plasma levels of uPA, uPAR, D-dimer,IL-6 and TNF-α in the patients with SIRS were obviously higher than those in the non-SIRS patients and controls (P<0.001). Correlation analysis showed a positive correlation between uPAR and IL-6 levels (r=0.395, P=0.004) and between uPAR and TNF-α levels (r=0.606, P<0.001), but no correlation between uPAR and D-dimer levels (r=0.069, P=0.632). No correlation was observed between uPA, D-dimer, IL-6 and TNF-α levels (P>0.05). The establishment of ROC curve was based on the levels of uPAR, D-dimer, IL-6 and TNF-α in 24 hours for the diagnosis of multiple organ dysfunction syndrome (MODS), and the ROC areas under the curve were 0.76, 0.58, 0.86 and 0.83, respectively.
CONCLUSIONS:
uPA and uPAR play a major role in patients with SIRS in the process of coagulation disorder, but the mechanism of SIRS is not the same. uPAR may play a central role in the development of SIRS to MODS.
KEY WORDS: Systemic inflammatory response syndrome, Multiple organ dysfunction syndrome, D-dimer, Interleukin-6, Tumor necrosis factor-alpha, Coagulant function
INTRODUCTION
Systemic inflammatory response syndrome (SIRS) is a critical illness caused by infection or non-infection factors. Uncontrolled release of inflammatory mediator and cytokines is the most important trait of SIRS. Coagulation can be activated directly or indirectly by these inflammatory mediators or cytokines, resulting in the imbalance between coagulation and anti-coagulation of the body.[1,2] The imbalance of fibrinolytic and anticoagulant in patients with SIRS indicates negtive consequences of the disease.[3] Urokinase type plasminogen activator (uPA) and its receptor (uPAR) are of physiological and pathological significance in many aspects, including local fibrinolysis, inflammation, matrix reconstruction, angiogenesis, tissue repair and cell migration.[4] The present study was to test the levels of uPA, uPAR, D-dimer (D-D), IL-6, and TNF-α in plasma, explore the correlation of uPA and uPAR with other cytokines like D-D, IL-6 and TNF-α and discuss their significance in SIRS and the process changing from SIRS to multiple organ dysfunction syndrome (MODS).
METHODS
Patients
In this prospective, case control study, samples were taken from patients who visited Wuhan Central Hospital for physical examination, or hospitalized patients and ICU patients during the period of 2008-2010 (35 patients with non-SIRS and 50 patients with SIRS). Thirty healthy people without evidence of infection served as controls. All of the patients were treated at the general medicine department of the hospital. They were diagnosed with SIRS according to the diagnostic criteria set up by the American Association of Chest Physicians and ICU Association in 1991.[5]
Methods
Ten mL fasting venous blood was taken from each patient in the morning every day. The blood was taken from the patients of the non-SIRS group the very day when they visited the hospital, and that from the patients of the SIRS group when SIRS occurred. Five mL blood was used to test D-D with ELISA. The remaining 5 mL blood was placed in a sodium citrate tube for isolation of plasma and red blood cells and finally stored at -70°C. The plasma was subjected to ELISA to detect the levels of uPA and uPAR. The levels of TNF-α and IL-6 were also determined with ELISA. The ELISA was made according to the instructions of the kit used (ADI, America).
Statistical analysis
The data were analyzed with statistics software SPSS 17.0. Measurement data were expressed as mean±SD. Comparison among different groups was performed by independent sample t test. Non-normal distribution of measurement data was taken as median (minimum, maximum), and the data were analyzed with Wilcoxon's rank-sum test and the Mann-Whitney U test. Pearson's produt-moment correlation coefficient analysis was applied to analyze the correlation. P<0.05 was considered statistically significant. A ROC curve was made with the values of uPA, uPAR, IL-6 and TNF-α within 24 hours, and the area under the curve was calculated to detect MODS according to the values of uPA, uPAR, IL-6 and TNF-α in the blood of SIRS patients.
RESULTS
Comparison of levels of uPA, uPAR, D-D, IL-6 and TNF-α between different groups
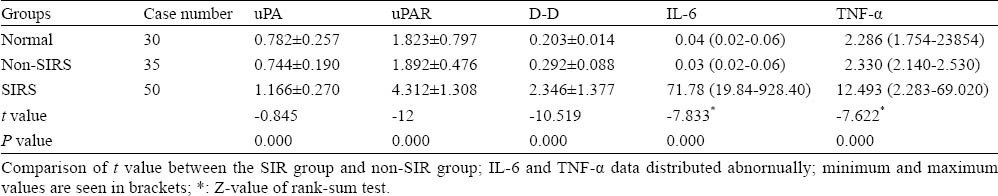
There were no significant differences in age and gender in all subjects (P>0.05) (Table 1). The levels of plasma uPA, uPAR, IL-6 and TNF-α in the non-SIRS group were not significantly different from those in the control group (P>0.05). However, the levels of plasma uPA, uPAR, IL-6 and TNF-α in the SIRS group were significantly higher than those in the control group and non-SIRS group (P< 0.001) (Table 2).
Table 1.
Subjects metadata (no.)
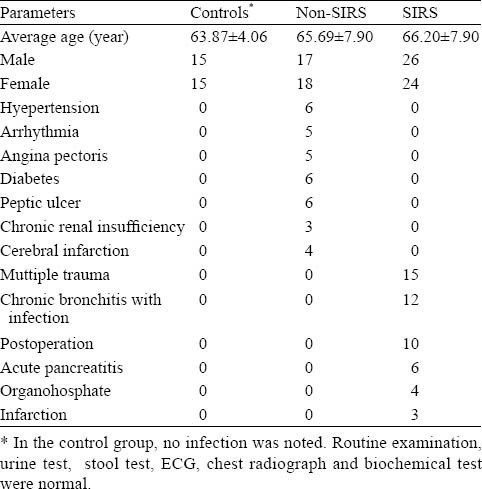
Table 2.
Comparison of uPA, uPAR, D-D, IL-6 and TNF-α concentration between the three groups (ng/mL)
Correlation analysis of levels of uPA, uPAR, D-D, IL-6 and TNF-α in the plasma of SIRS patients
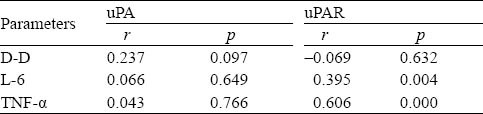
In the blood of the SIRS patients, the level of uPA was not correlated with the levels of DD, IL-6 and TNF-α (P>0.5). The level of uPAR was not correlated with the level of DD (r=−0.069, P=0.632), but it was positively correlated with the levels of IL-6 (r=0.395, P= 0.004) and TNF-α (r=0.606, P<0.001) (Table 3).
Table 3.
Correlation analysis of uPA, uPAR, D-D, L-6 and TNF-± in SIRS Patients
Levels of serum uPA, uPAR, IL-6 and TNF-α in detecting MODS in SIRS patients
In the SIRS patients, there were 24 patients with MODS. According to the ROC curve, the accuracy in diagnosing MODS with the levels of plasma uPAR, IL-6 and TNF-α in 24 hours was mild, and their AUROC values were 0.76, 0.86, and 0.83 seperately. However, the diagnostic accuracy was lower with the level of uPA (AUROC=0.58) in 24 hours (Figure 1).
Figure 1.
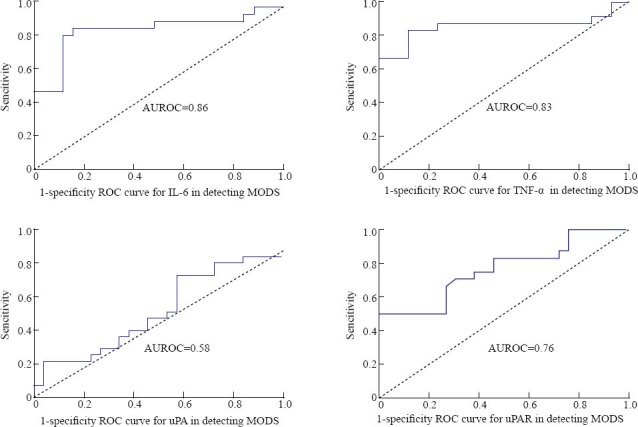
The levels of IL-6, TNF-α uPA, and uPAR in detecting MODS in SIRS patients.
DISCUSSION
SIRS is a symptom induced by infectious or non- infectious factors associated with imbalance of inflammation and antiinflammation. It is thought to be due to inflammation, coagulation and fibrinolysis.[7,8] D-D as a specific marker which stands for the process that plasma cross-linked fibrinogen is degraded by plasmase fibers has been accepted as an important indicator for the degradation of fibrinolysis.[9] In our study the level of D-D was significantly higher in the SIRS group than in the non-SIRS group and the control group, indicating that SIRS patients present with coagulation problem and hyperfibrinolysis. uPA is considered to be one of the media of fibrinolysis.[10] Whereas uPAR is a multifunctional receptor expressed on the surface of monocytes, neutrophils, eosinophils and activated T cells. Binding with its ligand uPA, uPAR shows local fibrinolysis and activates the conversion of plasminogen to plasmin, thus leading to the degradation of fibrin. [11] The level of uPA was reported to elevate in sepsis patients.[12,13] In the present study the levels of uPA and uPAR were higher in the SIRS group than in the control and non-SIRS groups, indicating that the overexpression of uPA and uPAR enhances fibrinolytic hyperactivity, which causes coagulopathy in the patient.
Excessively released cytokines in SIRS can activate the coagulation system in various ways, and form the complex response of coagulation, inflammatory mediators, cytokines and complement network.[14,15] In this study, the level of uPA was not related to the levels of IL-6 and TNF-α in the SIRS patients but the level of uPAR was positively correlated with the levels of IL-6 and TNF-α. These results indicate that uPA and uPAR are not consistent with the network of clotting and inflammation reaction of the SIRS patients. uPA plays a great role in the regulation of fibrinolytic and immune protection, whereas uPAR intervenes the immunity and inflammation by degradation of the extracellular matrix, and regulating cell adhesion, migration and regeneration. The local release of soluble uPAR (suPAR) also causes the aggregation and activation of inflammatory cells.[9] Compared with uPAR gene knockout mice, uPA gene knockout mice were found to have different susceptibility to different pathogen infections. It is speculated that uPA and uPAR play a role in the immune response via different steps and different ways.[16,17] Thus, compared with uPA, uPAR is associated with inflammatory mediators.
MODS is one of the leading causes of death of patients with grave disease. Amost all of MODS patients have SIRS in the development of the disease.[6] SIRS is thought to be an inevitable pathophysiological process in the development of MODS.[18] The disease will continue to progress and the body of SIRS patients will be damaged by new factors as a “second hit”. The second attack causes the waterfall-like release of inflammatory mediators until the appearance of tissue damage and organ dysfunction.[19] Inflammatory mediators play a great role in the development of SIRS to MODS. It has been confirmed that both IL-6 and TNF-α play a role in the development of SIRS.[20] In our study, a ROC curve was made for uPA, uPAR, IL-6 and TNF-α in the SIRS patients in 24 hours separately, and the results indicated that compared with IL-6 and TNF-α uPAR can also be used to diagnose MODS. We hypothesize that uPAR is essential in the development of SIRS to MODS. uPAR plays a central role in the proteolysis of endothelial cells surrounding the matrix.[9] A series of processes accelerate the occurrence of MODS in addition to the increase of vascular endothelial cell gap, destruction of endothelial integrity, interstitial edema, vascular leakage, etc. However, uPA has a lower ability to diagnose MODS than uPAR because of its different role in the process of MODS.
In conclusion, uPA and uPAR participate in the process of coagulation disorder in patients with SIRS. But the two factors have different roles in the development of SIRS. The increase of uPAR in blood may promote the development of SIRS to MODS.
Footnotes
Funding: None.
Ethical approval: The study was approved by the Ethics Committee of Wuhan Central Hospital.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subjects of this article.
Contributors: Li Y proposed and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Asakura H, Wada H, Okamoto K, Iba T, Uchiyama T, Eguchi Y, et al. Evaluation of haemostatic molecular markers for diagonosis of disseminated intravascular coagulation in patients with infections. Thromb Haemost. 2006;95:282–287. doi: 10.1160/TH05-04-0286. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen JS, Larsson A, Rix T, Nyboe R, Gjedsted J, Krog J, et al. The effect of activated protein C on plasma cytokine levels in a porcin model of acute endotoxemia. Intensive Care Med. 2007;33:1085–1093. doi: 10.1007/s00134-007-0631-1. [DOI] [PubMed] [Google Scholar]
- 3.Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van Aken H, De Vriese A, et al. The lectin-like domain of thrombomodulin confersprotection from neutrophil-2 mediated tissue damage by suppressing adhesionmolecule expression via nuclear factor kappa B and mitogen-activated protein kinase pathways. J Exp Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song SJ, Hu JB, Wang HX, Wen SQ, Ding MP, Huang JZ. The expression of urokinase-type plasminogen activator and its receptor in plasma of patients with cerebral infarction. Zhonghua Yi Xue Za Zhi. 2003;83:1583–1585. [PubMed] [Google Scholar]
- 5.American College Of Chest Physicians/ Society Of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failures and guidelines for the use of innovative therapies in sepsis. Critical Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 6.Wang Q, Liu D, Bai Y. T-cryptantigen (TCA) activation in sever pneumonia complicated with multiple organ failure. Transfus Apher Sci. 2010;43:361–364. doi: 10.1016/j.transci.2010.10.008. Epub 2010 Nov 13. [DOI] [PubMed] [Google Scholar]
- 7.Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011 May 12; doi: 10.1007/s10156-011-0254-x. [DOI] [PubMed] [Google Scholar]
- 8.Verhamme P, Hoylaerts MF. Hemostasis and inflammation: two of a kind? Thromb J. 2009;7:15. doi: 10.1186/1477-9560-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe Gd. How to search for the role and prevalence of defective fibrinolytic states as triggers of myocardial infaretion. The haemostasis epidmiologist's view. Ital Heart J. 2001;9:656–657. [PubMed] [Google Scholar]
- 10.Anna Mondino, Francesco Blasi. uPA and uPAR in fibrinolysis, immunity and pathology. Trends in Immunology. 2004;25:450–455. doi: 10.1016/j.it.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Blasi F, Carmeliet P. uPAR: a versatile signaling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 12.Robbie LA, Dummer S, Booth NA, Adey G, Bennett B. Plasminogen activator inhibitor 2 and urokinase-type plasminogen activator in plasma and leucocytes in patients with severe sepsis. Br. J. Haematol. 2000;109:342–348. doi: 10.1046/j.1365-2141.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- 13.Philippe J, Offner F, Declerk PJ, Leroux-Rouls G, Vogelaers D, Baele G, et al. Fibrinolysis and coagulation in patients with infectious disease and sepsis. Thromb Haemost. 1991;65:291–295. [PubMed] [Google Scholar]
- 14.Munzel T, Sinning C, Post F. Pathophysiology diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 15.Herzum I, Renz H. Inflammatory markers in SIRS sepsis and septic shock. Curr Med Chem. 2008;15:581–587. doi: 10.2174/092986708783769704. [DOI] [PubMed] [Google Scholar]
- 16.Rijneveld AW, Levi M, Florquin S, Speelman P, Carmeliet P, van Der Poll T. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J. Immunol. 2002;168:3507–3511. doi: 10.4049/jimmunol.168.7.3507. [DOI] [PubMed] [Google Scholar]
- 17.Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J Immunol. 2000;165:1513–1519. doi: 10.4049/jimmunol.165.3.1513. [DOI] [PubMed] [Google Scholar]
- 18.Makhoul Z, Kristal AR, Gulati R, Luick B, Bersamin A, Boyer B, et al. Associations of very high intakes of eicosapentaenoic and docosahexaenoic acids with biomarkers of chronic disease risk among Yup’ik Eskimos. Am J Clin Nutr. 2010;91:777–785. doi: 10.3945/ajcn.2009.28820. Epub 2010 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinsky MR. Sepsis and multiple organ failure. Contrib Nephrol. 2007;156:47–63. doi: 10.1159/000102070. [DOI] [PubMed] [Google Scholar]
- 20.Kohut M, Hartleb M, Hartleb T. Significance of serum concentrations of pro-and anti-inflammatory cytokines in identification of patients with Crohn's disease. Pol Merkur Lekarski. 2010;29:169–172. [PubMed] [Google Scholar]


