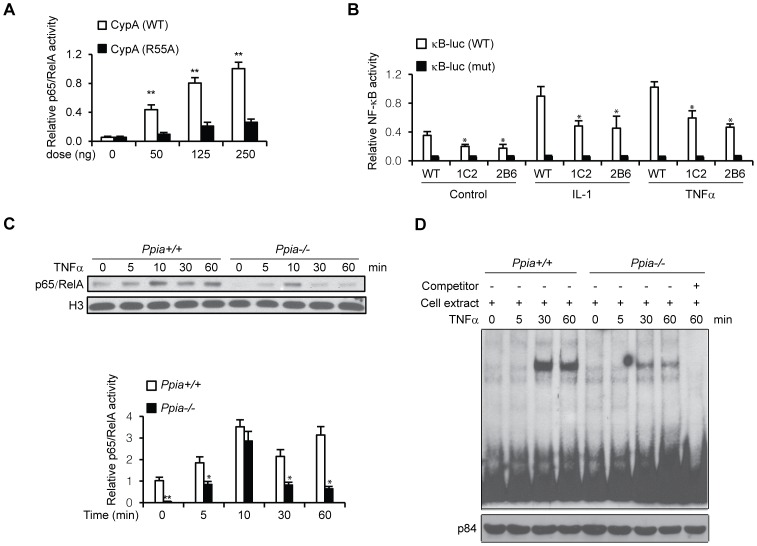
Figure 5. CypA is associated with NF-κB binding and transcriptional activities.
(A) CypA activates κB site-mediated luciferase activity in a dose-dependent manner. Hela cells were transfected with indicated amounts of CypA, together with pGL4 nf-κb-Luc reporter constructs for 24 hrs, followed by luciferase activity assays. CypA (R55A) was used as negative control. (B) TNF-α-induced nuclear p65 levels degraded more quickly in MEFs isolated from Ppia −/− mice. MEFs were treated with TNF-α for the indicated times, and nuclear extracts were prepared for Western blot analysis for p65 expression levels. Histone H3 protein served as an internal control. (C) Reduced NF-κB binding activity associated with CypA KO MEFs. EMSAs were performed with nuclear extracts from MEFs isolated from WT (Ppia +/+) and CypA KO (Ppia −/−) mice. NF-κB oligos were used as a probe. For competition experiments, nuclear extracts were pre-incubated with 100-fold molar excess of unlabeled oligos at 25°C for 10 min before addition of labeled probes. P84 was used as an internal loading control. (D) CypA is required for cytokine-induced full activation of κB-Luc activity. C2C12 cells and CypA Kd clones 1C2 and 2B6 were transfected with wild-type (WT) or mutant (MUT) pGL4 nf-κb-Luc reporter constructs, followed by treatment with IL-1α or TNF-α. Cells were harvested for luciferase assays.