Abstract
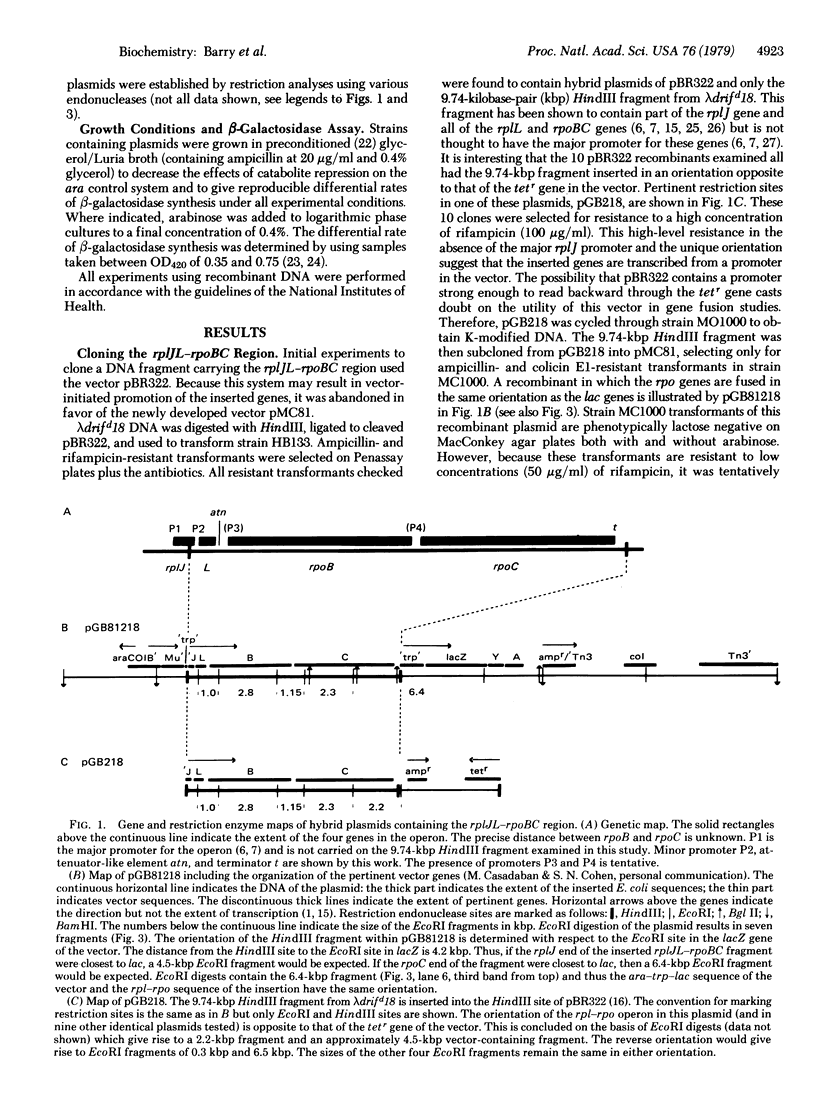
Gene fusions constructed in vitro have been used to examine transcription regulatory signals from the operon which encodes ribosomal proteins L10 and L7/12 and the RNA polymerase beta and beta' subunits (the rplJL-rpoBC operon). Portions of this operon, which were obtained by in vitro deletions, have been placed between the ara promoter and the lacZ gene in the gene-fusion plasmid pMC81 developed by M. Casadaban and S. Cohen. The effect of the inserted DNA segment on the expression of the lacZ gene (in the presence and absence of arabinose) permits the localization of regulatory signals to discrete regions of the rplJL-rpoBC operon. An element that reduces the level of distal gene expression to one-sixth is located on a fragment which spans the rplL-rpoB intercistronic region. This strongly supports the idea that there is an attenuator in this region. The terminator for the operon is located on a fragment which spans the 3' end of the rpoC gene. The major promoter for the operon precedes the rplJ gene [Yamamoto, M. & Nomura, M. (1978) Proc. Natl. Acad. Sci. USA 75, 3891-3895 and Linn, T. & Scaife, J. (1978) Nature (London) 276, 33-37] and was not examined in this study. However, a weak promoter is observed on the fragment that spans the rplJ-rplL intercistronic region. Other regions of the operon may also contain weak promoters. The contribution of these elements to the regulation of this complex operon is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Regulation of synthesis and activity of a mutant RNA polymerase in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5416–5420. doi: 10.1073/pnas.74.12.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Transcription patterns of adjacent segments on the chromosome of Escherichia coli containing genes coding for four 50S ribosomal proteins and the beta and beta' subunits of RNA polymerase. J Mol Biol. 1977 Oct 5;115(4):603–625. doi: 10.1016/0022-2836(77)90105-x. [DOI] [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- Goldberg G., Caldwell P., Weissbach H., Brot N. In vitro regulation of DNA-dependent synthesis of Escherichia coli ribosomal protein L12. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1716–1720. doi: 10.1073/pnas.76.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward R. S., Fyfe S. K. Non-coordinate expression of the neighbouring genes rplL and rpoB,C of Escherichia coli. Mol Gen Genet. 1978 Mar 20;160(1):77–80. doi: 10.1007/BF00275121. [DOI] [PubMed] [Google Scholar]
- Hayward R. S., Tittawella I. P., Scaife J. G. Evidence for specific control of RNA polymerase synthesis in Escherichia coli. Nat New Biol. 1973 May 2;243(122):6–9. [PubMed] [Google Scholar]
- Iwakura Y., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. II. control of RNA polymerase synthesis during nutritional shift up and down. Mol Gen Genet. 1975 Dec 23;142(1):67–84. [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Burgess R. R., Nomura M. Identification of a gene for the alpha-subunit of RNA polymerase at the str-spc region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5036–5040. doi: 10.1073/pnas.72.12.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl S., Yamamoto M., Nomura M. Mapping of a cluster of genes for components of the transcriptional and translational machineries of Escherichia coli. J Mol Biol. 1977 Jan 5;109(1):23–47. doi: 10.1016/s0022-2836(77)80044-2. [DOI] [PubMed] [Google Scholar]
- Linn T., Scaife J. Identification of a single promoter in E. coli for rplJ, rplL and rpoBC. Nature. 1978 Nov 2;276(5683):33–37. doi: 10.1038/276033a0. [DOI] [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- Maher D. L., Dennis P. P. In vivo transcription of E. coli genes coding for rRNA, ribosomal proteins and subunits of RNA polymerase: influence of the stringent control system. Mol Gen Genet. 1977 Oct 20;155(2):203–211. doi: 10.1007/BF00393161. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Newman A. J., Linn T. G., Hayward R. S. Evidence for co-transcription of the RNA polymerase genes rpoBC with a ribosomal protein gene of escherichia coli. Mol Gen Genet. 1979 Jan 31;169(2):195–204. doi: 10.1007/BF00271671. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Post L. E., Arfsten A. E., Reusser F., Nomura M. DNA sequences of promoter regions for the str and spc ribosomal protein operons in E. coli. Cell. 1978 Sep;15(1):215–229. doi: 10.1016/0092-8674(78)90096-x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL H. R. SYNTHESIS OF BETA-D-GALACTOSIDASE AFTER F-DUCTION OF LAC+GENES INTO ESCHERICHIA COLI. J Mol Biol. 1965 Jan;11:23–34. doi: 10.1016/s0022-2836(65)80168-1. [DOI] [PubMed] [Google Scholar]
- Schweitzer S. M., Matzura H. Transformation of Escherichia coli by a specific DNA restriction fragment. Mol Gen Genet. 1977 Oct 20;155(2):213–217. doi: 10.1007/BF00393162. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R. Copies of proteins L7 and L12 and heterogeneity of the large subunit of Escherichia coli ribosome. J Mol Biol. 1975 Jun 15;95(1):1–8. doi: 10.1016/0022-2836(75)90330-7. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Platt T. Transcription termination: nucleotide sequence at 3' end of tryptophan operon in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5442–5446. doi: 10.1073/pnas.75.11.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Nomura M. Contranscription of genes for RNA polymerase subunits beta and beta' with genes for ribosomal proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3891–3895. doi: 10.1073/pnas.75.8.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]



