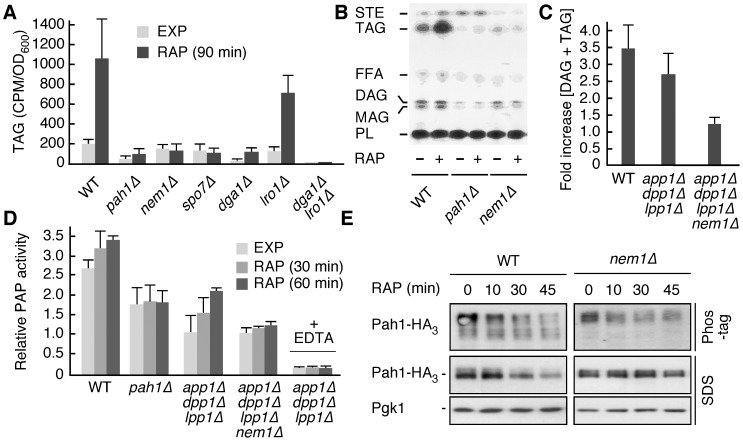
Figure 1. TORC1 inhibition activates Pah1 phosphatidate phosphatase via the Nem1-Spo7 protein phosphatase module.
(A) Incorporation of radioactively labeled palmitic acid into triacylglycerol (TAG) was monitored in exponentially growing (EXP) and rapamycin-treated (RAP; 90 min) cells. Relevant genotypes of strains are indicated (WT, wild type). (B) Representative TLC plate showing radioactively-labeled, separated lipid samples from the experiment in (A) that were extracted from exponentially growing (RAP; −) and rapamycin-treated (RAP; +) WT, pah1Δ, and nem1Δ strains. STE, steryl esters; FFA, free fatty acids; DAG, diacylglycerol; MAG, monoacylglycerol; PL, phospholipids. (C) The combined levels of DAG and TAG were determined in rapamycin-treated (4 h) cells using a commercially available enzymatic kit and expressed in each case relative to the respective levels in exponentially growing cells. (D) Relative PAP activity in exponentially growing (EXP) and rapamycin-treated (RAP; 30 min and 60 min) cells. Results are presented as relative activities compared to the activity in exponentially growing app1Δ dpp1Δ lpp1Δ cells (defined as 1.0), which express Pah1 as only source of PAP activity [44]. Assays carried out in the presence of EDTA are indicated (+ EDTA). (E) Phos-tag phosphate-affinity gel electrophoresis and SDS-PAGE analyses of endogenously tagged Pah1-HA3 in exponentially growing WT and nem1Δ cells treated with rapamycin (RAP) for the indicated times. The levels of Pgk1 served as loading controls. In Figures 1A, 1C, and 1D, each bar represents the mean ± SD of three experiments.