Abstract
Background/aims
Alterations in noradrenergic (NE) signaling have been implicated in the pathophysiology of irritable bowel syndrome (IBS), and adrenergic receptors are potential treatment targets.
Methods
To characterize central NE signaling in IBS, 11 patients and 11 healthy controls (HCs) were studied 3 times during an auditory oddball vigilance task after double-blind ingestion of the α2-adrenoreceptor (α2AR) antagonist yohimbine (YOH), the α2AR agonist clonidine (CLO), or placebo (PLA). Regional cerebral glucose metabolism was measured with [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET). Measures of anxiety, early-life trauma, plasma NE and blood pressure were acquired.
Results
Patients had higher plasma NE levels than HCs before and after ingestion of all drugs (all p <0.05). YOH increased plasma NE and more anxiety in patients than in HCs. After YOH, NE levels directly correlated with drug-induced increases in anxiety in IBS patients (r=0.61), but not in HCs. IBS patients showed less YOH-mediated reduction of activity in a central arousal circuit, consistent with fewer functional presynaptic α2AR. In HCs, but not in patients, activation of amygdala and subgenual anterior cingulate cortex (sgACC) was inversely correlated with activation of anterior mid cingulate cortex (aMCC), and state anxiety covaried directly with activity in limbic and right frontotemporal cortices, but indirectly with activity in the left frontotemporal cortex. YOH-mediated reduction of activity in brainstem and amygdala inversely correlated with early life trauma.
Conclusions
IBS patients showed evidence for increased noradrenergic activity consistent with downregulation of presynaptic inhibitory α2ARs. Activity within central arousal circuits was biased toward greater excitability and reduced corticolimbic inhibition in IBS. Early life trauma may be one mediator of these abnormalities.
Keywords: Yohimbine, α2 Adrenergic receptors, Early-life trauma, Corticolimbic inhibition
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder characterized by recurrent abdominal pain associated with alterations in bowel habits (Longstreth et al., 2006). Symptom-related anxiety, and elevated trait anxiety are seen in the majority of affected patients (Tillisch et al., 2012), and the prevalence of disorders of mood and affect is more common both in patients, and non-healthcare seeking individuals meeting symptom criteria (Naliboff, 2009). A proposed neurobiological model of IBS shares considerable features with analogous models proposed for mood/affect disorders (Kennedy et al., 2012), and fibromyalgia (Verdu et al., 2008). A central feature of these models is upregulation of central arousal circuits involving noradrenergic (NE) and corticotropin releasing factor (CRF) signaling (Dickhaus et al., 2003; Hubbard et al., 2011). Analogous changes have been reported in animals with early life trauma (Al-Chaer and Weaver, 2009). In fact a history of aversive early life events, or a history of physical or sexual trauma, greatly enhances an individual's vulnerability for stress-related conditions such as IBS. Thus the likelihood of developing IBS in life is higher in patients with such aversive early life events as loss of the primary caregiver, divorce of their parents, or abuse (Al-Chaer and Weaver, 2009).
In preclinical models, alterations in NE pathways originating in the locus coeruleus complex (LCC) of the dorsal pons are implicated in IBS pathophysiology (Martinez and Taché, 2006), and recent studies have shown that antagonism of the CRF receptor type 1 (CRF-R1) is able to attenuate LCC responsiveness to a stressor in IBS (Hubbard et al., 2011). Enhanced perception of visceral signals may involve compromised ability to engage endogenous (descending) NE antinociceptive mechanisms (Chang et al., 2000b) and symptom-related anxiety and hypervigilance (Chang et al., 2000a; Coull et al., 2000; Naliboff et al., 1997), and may involve central (ascending) hyperarousal (Arnsten and Goldmanrakic, 1984; Valentino et al., 1999). However, few prior IBS studies directly assess central NE abnormalities in humans, let alone IBS patients.
NE released from ascending fibers interacts with 3 families of adrenergic receptors, the stimulatory α1 and β adrenoreceptors, and inhibitory α2 adrenoreceptor. NE has the highest affinity for α2 receptors, which have 3 subtypes. α2A and α2C receptors are predominantly presynaptic, although all 3 are found postsynaptically (Ramos and Arnsten, 2007). α2A receptor agents have been used as pharmacological probes to presynaptically modulate central NE release. The α2A receptor antagonist Idazoxan increased hippocampal NE release in stressed animals (Lim et al., 2010). Peripheral YOH stimulated NE and CRF releases, increased fear-potentiated startle (Bijlsma et al., 2010) and accentuated fear and anxiety (Meyerbroeker et al., 2012; Soeter and Kindt, 2011, 2012). Anxiety is the most common adverse effect of YOH.
Many IBS patients have subclinically elevated (Naliboff, 2009) and symptom-related anxiety (Labus et al., 2004), and there is considerable co-morbidity with anxiety disorders with evidence for increased central NE (Wong et al., 2000). In animal models, short-term chronic social stress downregulated α2A adrenoreceptors (Flugge et al., 2003) in areas suggesting increased NE release. Dysregulations as a result of stressful levels such as early life trauma, increase the risk for the development of mood/affect disorders and persistent pain syndromes. In animals, it has been associated with downregulation of presynaptic α2 adrenoreceptors, and increased sympathetic responsiveness (Ladd et al., 2000). Pharmacological evidence for downregulation of central α2A adrenoreceptors in IBS has been demonstrated by desipramine challenge, which is thought to release growth hormone via postsynaptic hypothalamic α2 receptors (Dinan et al., 1990). More recently, α2 adrenoreceptor polymorphisms have been associated with IBS and somatization (Bremner et al., 1996a, 1996b). Preliminary clinical results support a possible therapeutic role for the α2 adrenoreceptor agonist CLO(Camilleri et al.,2003).
We assessed whether IBS patients while performing a vigilance task show NE abnormalities related to early life trauma, increased anxiety, and hypervigilance toward potentially aversive stimuli by quantifying effects of adrenergic agents on peripheral NE, mood, and relative regional glucose metabolism, an index of local brain activity. In previous resting-state studies, neocortical metabolism decreased after YOH-induced NE increase, and increased after CLO-induced NE decrease (Bremner et al., 1997). By using YOH as a probe of central noradrenergic signaling, we specifically asked if IBS patients show: 1) evidence for a downregulation of presynaptic α2A adrenoreceptors, and increased central NE release, as indexed by smaller reductions in brain metabolism, 2) altered connectivity within central arousal circuits, 3) correlation of these effects with early life trauma due to upregulation of arousal circuits involving NE and CRF common in early traumatic effects which in turn are prevalent among IBS, and 4) brain and behavioral responses to CLO in order to assess both agonists and antagonists in the same study.
Materials and methods
Participants
Eleven IBS patients meeting Rome II criteria (6 male; mean age: 40.5 sd 12.9; range 21–60) and age and sex matched HCs (mean age: 37.3 sd 10.6) recruited by local advertisements completed the study. Rome II diagnostic criteria for IBS in this study were based on the definition of recurrent abdominal pain or discomfort for at least 3 days/month in the last 3 months and associated with two or more of the following: 1. improvement in defecation, 2. onset associated with a change in frequency of stool and 3. onset associated with a change in form (appearance) of stool. Structured clinical interviews were conducted and subjects were excluded if they had any major psychiatric disorders, including any anxiety and depression spectrum disorders within the past year, alcohol/substance abuse within two years, serious medical conditions, metal implants, or hearing loss. Although no drug screening was performed, subjects were excluded from the study if they were taking any medications except in the case of mild tricyclic antidepressants (TCAs) so long as there were no changes in the past 3 months. Informed consents were obtained from all subjects as approved by the Institutional Review Board at the Veterans Administration Greater Los Angeles Healthcare System and the University of California Los Angeles.
Questionnaires
During screening, patients completed the UCLA Bowel Symptom Questionnaire (BSQ) (Munakata et al., 1997), the Eysenck Personality Questionnaire-Revised (EPQR) (Eysenck and Eysenck, 1994), the Visceral Sensitivity Index (VSI) (Labus et al., 2007), the Hospital Anxiety and Depression Index (HAD) (Farhadi et al., 2001), and the Early Trauma Inventory Short Form (ETISF) (Bremner et al., 2007). The ETISF quantifies life traumas experienced before age 18 within four domains: general, physical, emotional and sexual. A summary score formed by adding the subscale scores measured overall childhood trauma. On scan days, subjects rated fatigue and anxiety levels using the Stress Symptom Rating scales (SSR) before and after drug administration (Naliboff et al., 1991).
Drugs
Yohimbine tablets at 40 mg were manufactured by the Clinical Research Pharmacy Coordinating Center, VA Cooperative Studies Program (Albuquerque, New Mexico). Placebo capsules were obtained through the professional Arts Pharmacy (Baltimore, MD). Clonidine tablets at 0.2 mg were on formulary at the VA Greater Los Angeles Healthcare System Pharmacy, which stored and dispensed all study drugs/placebo.
Study paradigm
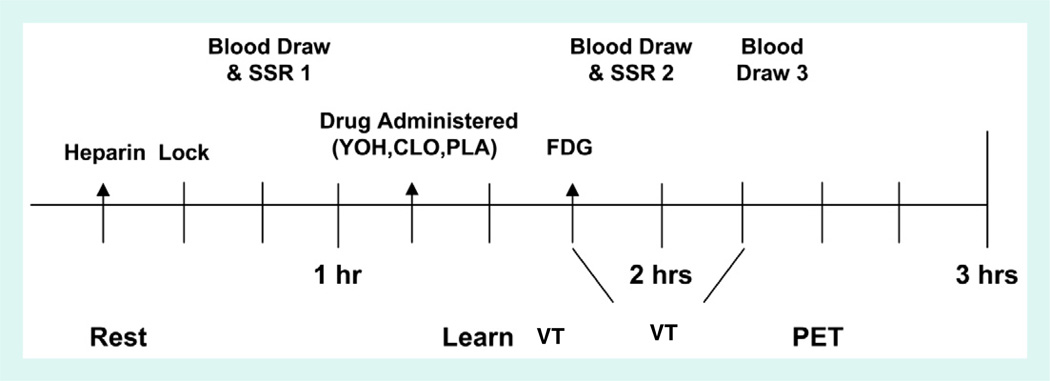
The health assessment was followed by three drug sessions (median = 12±12 days apart) detailed in Supplementary Fig. S1. Groups were matched for the order of orally-administered identical (double-blind) tablets containing YOH, CLO and placebo (PLA). At each drug session, after fasting for 4 h (no alcohol for 24 h), subjects underwent a modified vigilance oddball task while blood pressure and EEG activity were recorded as previously described (Berman et al., 2002b). Preliminary EEG results have been reported (Berman et al., 2007). Thirty minutes after drug administration, participants received an intravenous injection containing 5 mCi of 2-[18F]-fluoro-2-deoxy-d-glucose (FDG). Immediately thereafter, subjects began four 6-minute auditory oddball task blocks (Berman et al., 2002b). Subjects were first instructed in the task and completed a practice block. Each vigilance block required fixation on a small central cross on a computer monitor during a 5-minute period during which 128 tones (100 ms 90 dB SPL) varying randomly in pitch (1000 or 2000 Hz) and ear (right or left) were presented via air conduction earphones (Eartone type 3A). Intermixed with the tones, 16 single visual words were presented above the fixation cross for 200 ms. Half of the words were related to IBS symptoms. The others were matched for initial letter, length and frequency in print. Results from this part of the study were reported elsewhere (Vianna et al., 2009).
Inline Supplementary Fig. S1 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2012.08.028.
Positron emission tomography (PET)
Participants were then positioned in a GE Advance NXi PET camera and a 5-minute transmission scan was done as previously reported (Berman et al., 2002a). Emission data were acquired for 20 min and reconstructed using the OSEM method to generate an image of relative glucose metabolism during the auditory–visual vigilance task performance. A structural MR1 was acquired using a 1.5 Tesla Siemens Sonata.
Norepinephrine assays
NE assays were performed using a commercially-available Extraction and EL1SA kit (BA 10–0200, Rocky Mountain Diagnostics, Inc.; Colorado Springs, CO). The limit of detection for this assay was 44 pg/ml and intra- and inter-assay precisions were less than 16.1% and 15%, respectively.
Effects of each drug on NE, blood pressure and self-rated mood were quantified by within subject t-tests. Group differences were assessed with between-subject t-tests. All blood samples were lost for one control subject, and all values registered below the lowest level of detect-ability (about 50 ppm) for one patient, so these two individuals could not be included in the analyses of plasma NE. Levene's test for the homogeneity of variances was applied to examine variability in plasma levels of NE. Given violations in the assumption of equal variance, Welch's t test, an adaptation of the t-test when samples may have unequal variance, was applied to test for differences in mean plasma levels (Welch, 1947). Postdrug plasma NE levels were computed by averaging assays taken at 60 and 90 min after drug administration.
Data analysis
PET analysis
Each subject's PET image was co-registered to their anatomical MRI. Statistical Parametric Mapping software (SPM5: http://www.fil.ion.ucl.ac.uk/spm/software/spm5/) normalized all images into a standardized space (Montreal Neurological Institute), and applied the general linear model to identify regions where relative activity differed by group (IBS, Control), drug (YOH, CLO, PLA) and their interaction. Statistical parametric maps were thresholded at p<0.005 (uncorrected). To increase statistical power in areas of a priori interest, we used a region-of-interest (ROI) strategy focusing upon eight structures associated with pain or its modulation: anterior (aINS) and posterior insula (pINS), anterior cingulate gyrus (ACC) – divided into subgenual (sgACC), pregenual (pgACC), and mid cingulate (MCC) subdivisions, amygdala, dorsal brainstem, and ventrolateral prefrontal cortex (vIPFC). Preliminary evidence from other structures was corrected for the whole-brain volume. An alpha level of p<0.05 after volume correction was adopted, with p<0.1 findings noted as trends.
The general linear model was also applied to quantify how brain activity covaried with 1) plasma NE and anxiety, 2) activity within 4 mm radius spheres centered at 26, −6, −18 (amygdala) and − 4, 24, −10 (sgACC), and 3) childhood trauma score (for mean-corrected YOH-minus-PLA subtraction images). One IBS patient and five HCs were not included in the final analysis due to missing trauma scores.
Results
Patient characteristics
Subject characteristics are shown in Inline Supplementary Table S1 Patients rated their symptom severity as mild (n = 3), moderate (n = 2) or severe (n = 6), and had an average symptom duration of 14.9 years. The average 24 h severity rating was 10.8 (range 3–17) on a scale from 0 to 20. Patients had a higher symptom related anxiety (VS1) (p<0.0001), and neuroticism (trait anxiety) scores (p =0.009). State anxiety and depression scores (all subclinical) did not differ between groups. One-tailed t-tests for groups with unequal variance suggested greater childhood trauma in IBS patients on the total score (p = 0.054) and physical trauma subscale (p =0.002).
Inline Supplementary Table S1 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2012.08.028.
Autonomic, NE and behavioral assessments
Autonomic
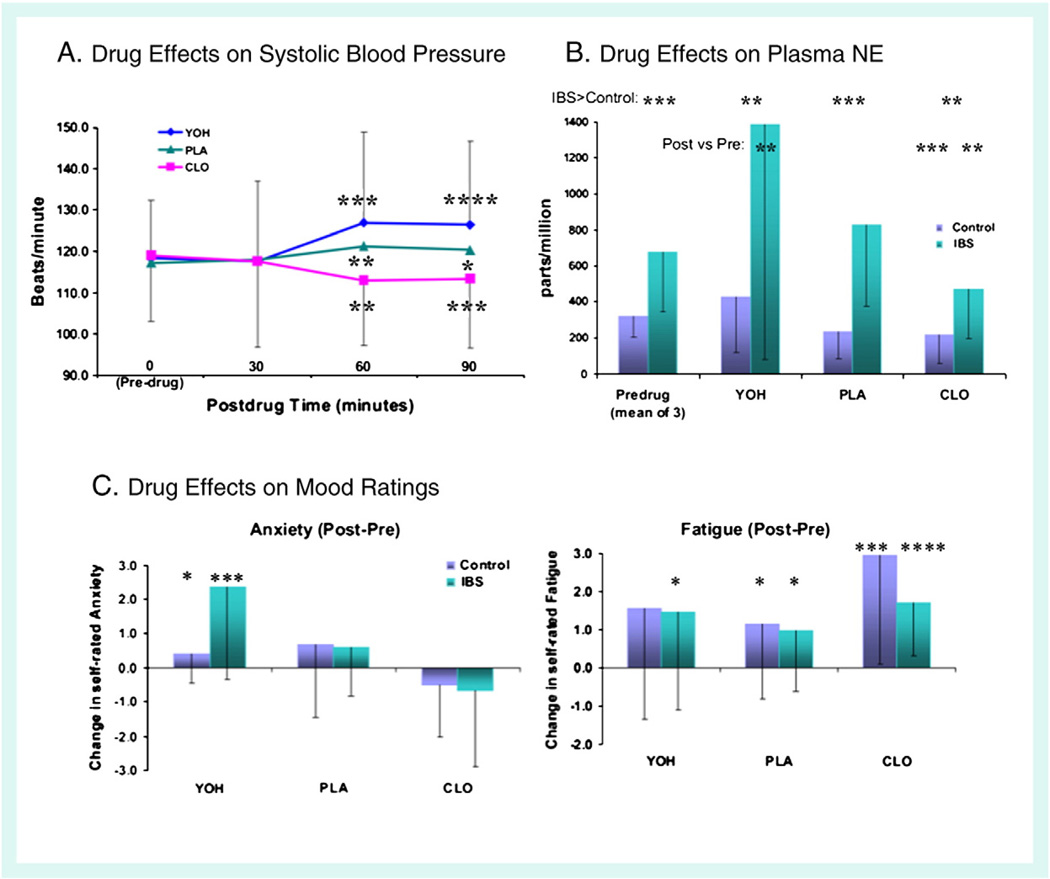
Predrug systolic blood pressure did not differ between patients (118±15) and HCs (119±11). Systolic blood pressure 30 min after drug administration did not differ from predrug values. At 60 and 90 min, across all participants, blood pressure decreased after CLO, increased slightly after PLA, and increased markedly after YOH (Inline Supplementary Fig. S2A). There were no group differences in systolic blood pressure post-drug.
NE assays
Pre-drug NE levels were more variable in IBS, F = 0.115, p = 0.002, and mean plasma level was significantly higher by Welch's t test (t (20) = 3.22, p =0.004, 678±334 vs. 319 ±113). For all participants, post drug NE levels were higher after YOH (mean±SD = 907±1047), and lower after CLO (344±252), as compared to PLA (532±448; both p<0.04). Plasma NE levels, which were higher in patients than in HCs prior to drug administration, were also higher after the administration of all three study drugs (Inline Supplementary Fig. S2B). The increase in plasma NE following YOH ingestion was larger in patients than in HCs (t = 1.83, p = 0.048).
Anxiety and fatigue
Although patients reported more self-rated fatigue (4.62 ± 2.02 vs. 3.31 ±1.90) state anxiety (2.51 ±1.45 vs. 1.64±1.19) prior to drug ingestion, these differences did not attain significance (both p>0.1). Fatigue increased after the administration of all three drugs (all p<0.01), but more after CLO than PLA (p = 0.02). No group differences in fatigue were observed post-drug.
Across groups (n = 22), self-rated anxiety increased after administration of YOH (p = 0.004), but not after PLA or CLO (both p>0.1). YOH increased anxiety more in IBS patients than in HCs (t = 2.28, p = 0.042). While anxiety prior to drug ingestion did not differ, anxiety post-YOH was higher in IBS patients than in HCs. (t = 2.97, p = 0.011). After ingestion of YOH, plasma NE level was directly correlated with drug-related anxiety increase in IBS patients (r = 0.61; p<0.01***), but not in HCs (r=−0.53; p<0.1*). Drug effects on self-rated anxiety and fatigue are shown in Inline Supplementary Fig. S2C
Inline Supplementary Fig. S2 can be found online at http://dx.doi.org/10.1016/j.neuroimage.2012.08.028.
Drug effects on brain activity
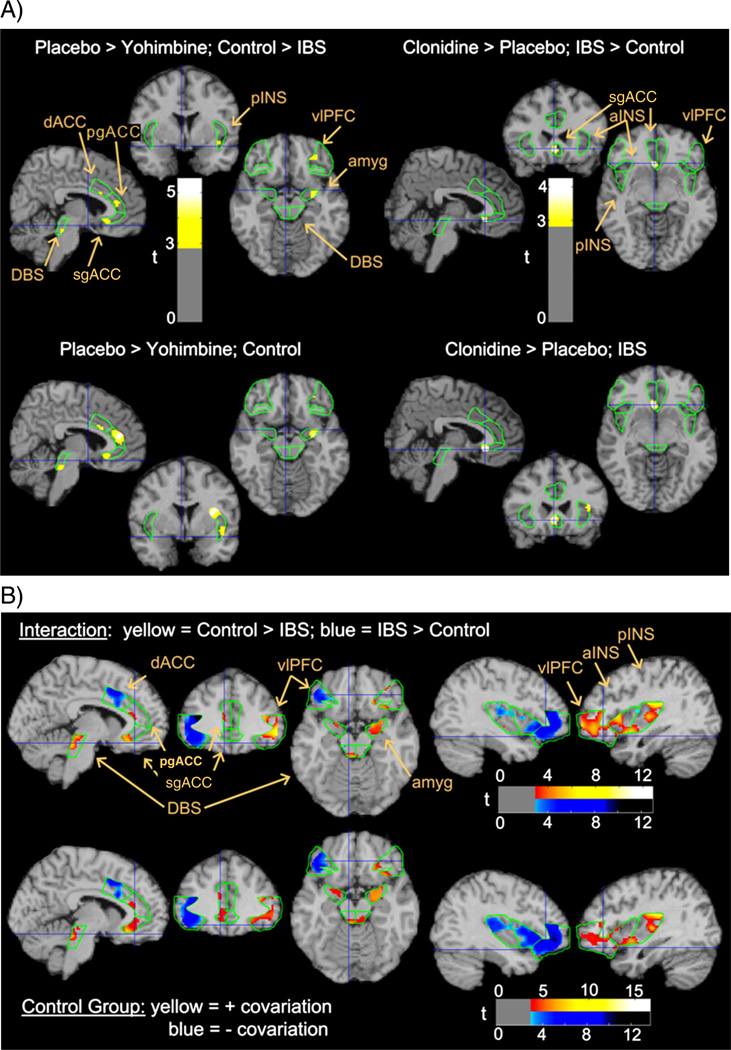
Across both groups, CLO was associated with more relative brain activity than YOH. Compared to PLA, CLO was also associated with more activity in most regions, and YOH with less activity in pgACC. However, YOH compared to PLA reduced brain activity in the ACC, amygdala, dorsal brainstem and pINS more in HCs than in patients. CLO increased activity in the sgACC more in patients than in HCs (Table 1 and Fig. 1).
Table 1.
Effects of drugs and IBS on brain activity. Group×drug interactions (individual group effects needed for interpretation).
| Cluster p | k | Voxel p (FWE) | T | X | y | z | ||
|---|---|---|---|---|---|---|---|---|
| PLA>YOH; HC>IBS | ||||||||
| Dorsal brainstem | L | 0.066 | 13 | 0.060 | 3.05 | −8 | −30 | −22 |
| R | NS | NS | ||||||
| Amygdala | L | NS | NS | |||||
| R | 0.038 | 32 | 0.001 * | 4.94 | 28 | −6 | −16 | |
| Subgenual ACC | L | 0.028 | 60 | 0.005 | 4.22 | −2 | 24 | −10 |
| R | 0.031 | 50 | 0.010 | 3.88 | 8 | 42 | −10 | |
| Pregenual ACC | L | 0.058 | 48 | 0.046 | 3.50 | −6 | 36 | 14 |
| R | NS | NS | ||||||
| Posterior insula | L | NS | NS | |||||
| R | 0.087 | 38 | 0.069 | 3.41 | 38 | 0 | −10 | |
| HC:PIA>YOH | ||||||||
| Dorsal brainstem | L | 0.035 | 42 | 0.037 | 3.29 | −8 | −30 | −24 |
| R | NS | NS | ||||||
| Amygdala | L | NS | NS | |||||
| R | 0.026 | 51 | <0.0005 * | 5.07 | 28 | −6 | −16 | |
| Subgenual ACC | L | 0.027 | 62 | 0.005 | 4.21 | −2 | 24 | −10 |
| R | 0.019 | 79 | 0.002 * | 4.44 | 10 | 42 | −10 | |
| Pregenual ACC | L | 0.001* | 376 | 0.001 * | 5.13 | −4 | 38 | 14 |
| R | 0.053 | 51 | 0.055 | 3.40 | 16 | 44 | 14 | |
| Posterior insula | L | 0.081 | 53 | 0.055 | 3.62 | −38 | −16 | −6 |
| R | 0.050 | 70 | 0.050 | 3.56 | 38 | 4 | 16 | |
| CLO>PIA, IBS>HC | ||||||||
| Subgenual ACC | L | 0.045 | 34 | 0.008 | 4.01 | −4 | 24 | −8 |
| R | NS | NS | ||||||
| IBS: CLO>PLA | ||||||||
| Subgenual ACC | L | 0.023 | 73 | 0.001 * | 4.74 | −4 | 24 | −10 |
| R | 0.028 | 55 | 0.007 | 4.01 | 10 | 40 | −12 | |
significant after Bonferroni correction, 0.05/16 = 0.0031, k = # of voxels. Con = control, PLA = placebo, YOH = yohimbine, FWE = family wise error corrected, ACC = anterior cingulate cortex, PFC = prefrontal cortex, NS=p>0.1.
Fig. 1.
Drug and group effects on relative glucose metabolism. Upper panel depicts p<0.005 statistical interactions in the regions of interest (ROIs – outlined in green) between group and drug effects (compared to placebo), superimposed on a structural MRI representing atlas space. Lower panel depicts individual group effects responsible for the interaction above. Other possible interactions and contributing group effects were insignificant. Yohimbine effects are depicted on slices 6 mm to the left, 2 mm anterior, and 14 mm inferior to the anterior commissure (i.e., MNI coordinates −6, 2, −14). Clonidine effects are depicted at MNI −4, 24, −8. All figures depict neurological orientation (left=left). sgACC = subgenual cingulate, pgACC = pregenual cingulate, MCC = mid cingulate cortex, aINS = anterior insula, pINS = posterior insula, amyg = amygdala, DBS = dorsal brainstem, and vlPFC = ventrolateral prefrontal cortex.
Relationship between plasma NE and state anxiety with brain activity
NE, an inhibitory neurotransmitter, correlated negatively with activity in most of the brain. There were no group differences in the magnitude of this effect. In contrast, salient group differences in the relationship of anxiety with regional brain activity indicated that in HCs, but not in IBS patients, anxiety covaried positively with activity in limbic structures and the right frontotemporal cortex, and covaried negatively with activity in the left frontotemporal cortex (Table 2 and Fig. 2).
Table 2.
Group differences in covariation of regional activity with anxiety.
| Cluster p | k | Voxel p (FWE) | T | X | Y | z | ||
|---|---|---|---|---|---|---|---|---|
| HC>IBS | ||||||||
| Dorsal brainstem | L | 0.007 | 142 | <0.0005 * | 6.33 | −2 | −36 | −18 |
| R | 0.036 | 41 | 0.001 * | 4.75 | 4 | −34 | −20 | |
| Amygdala | L | 0.070 | 10 | 0.006 | 4.09 | −18 | 0 | −18 |
| R | 0.003 * | 193 | <0.0005 * | 5.68 | 28 | 4 | −22 | |
| Subgenual ACC | L | 0.016 | 92 | <0.0005 * | 5.34 | −4 | 28 | −12 |
| R | NS | NS | ||||||
| Pregenual ACC | L | 0.046 | 60 | 0.031 | 3.76 | −10 | 36 | 16 |
| R | NS | 16 | 0.005 | 4.54 | 14 | 32 | 22 | |
| Anterior insula | L | NS | NS | |||||
| R | <0.0005 * | 547 | <0.0005 * | 6.83 | 36 | 20 | 8 | |
| Posterior insula | L | NS | 11 | 0.081 | 3.50 | −40 | −22 | −4 |
| R | 0.000 * | 554 | <0.0005 * | 7.51 | 36 | −22 | 20 | |
| Ventrolateral PFC | L | NS | NS | |||||
| R | <0.0005 * | 991 | <0.0005 * | 7.86 | 42 | 28 | 10 | |
| Positive covariation in the HC group | ||||||||
| Dorsal brainstem | L | 0.010 | 102 | <0.0005 * | 7.73 | −2 | −36 | −18 |
| R | 0.043 | 31 | <0.0005 * | 7.23 | 16 | −22 | −6 | |
| Amygdala | L | 0.021 | 58 | 0.002 * | 5.58 | −16 | −2 | −20 |
| R | 0.002 * | 218 | <0.0005 * | 7.85 | 26 | 2 | −24 | |
| Subgenual ACC | L | 0.005 | 146 | <0.0005 * | 7.24 | −4 | 28 | −12 |
| R | NS | NS | ||||||
| Pregenual ACC | L | 0.037 | 66 | 0.023 | 4.60 | −12 | 36 | 18 |
| R | NS | NS | ||||||
| Anterior insula | L | NS | NS | |||||
| R | 0.004 | 208 | <0.0005 * | 8.32 | 36 | 20 | 12 | |
| Posterior insula | L | NS | NS | |||||
| R | 0.003 * | 236 | <0.0005 * | 12.02 | 32 | −18 | 8 | |
| Ventrolateral PFC | L | NS | NS | |||||
| R | <0.0005 * | 974 | <0.0005 * | 9.18 | 40 | 26 | 10 | |
| Negative covariation in the IBS group | ||||||||
| Posterior insula | L | NS | NS | |||||
| R | NS | 12 | 0.037 | 4.47 | 48 | −8 | 0 | |
| IBS>HC | ||||||||
| MCC | L | 0.009 | 200 | <0.0005 * | 5.79 | −6 | 16 | 42 |
| R | NS | 23 | 0.020 | 4.14 | 12 | 26 | 38 | |
| Anterior insula | L | <0.0005 * | 489 | <0.0005 * | 8.25 | −36 | 30 | −8 |
| R | NS | NS | ||||||
| Posterior insula | L | 0.007 | 229 | 0.001 * | 5.58 | −44 | −20 | 12 |
| R | NS | NS | ||||||
| Ventrolateral PFC | L | <0.0005 * | 1204 | <0.0005 * | 11.03 | −36 | 36 | 12 |
| R | NS | NS | ||||||
| Negative covariation in the HC group | ||||||||
| MCC | L | 0.008 | 179 | 0.005 | 6.26 | −10 | 18 | 42 |
| R | 0.057 | 61 | 0.021 | 6.10 | 12 | 20 | 40 | |
| Anterior insula | L | <0.0005 * | 678 | <0.0005 * | 9.05 | −36 | 30 | −8 |
| R | NS | NS | ||||||
| Posterior insula | L | <0.0005 * | 553 | <0.0005 * | 7.82 | −42 | −22 | 20 |
| R | NS | NS | ||||||
| Ventrolateral PFC | L | <0.0005 * | 1299 | <0.0005 * | 9.97 | −38 | 46 | −4 |
| R | NS | NS | ||||||
significant after Bonferroni correction, 0.05/16 = 0.0031, k = # of voxels.
FWE = family wise error corrected, ACC = anterior cingulate cortex, PFC = prefrontal cortex, and MCC = mid cingulate cortex.
Fig. 2.
Covariance of relative glucose metabolism with self-rated anxiety by group. Upper panel depicts areas of p<0.005 statistical interaction in the ROIs between the slopes of covariation between metabolism and anxiety in the two groups. Lower panel depicts positive and negative covariations with anxiety in the control group, the major contributor to these interactions (see Table 2 for effects, Fig. 1 for format and abbreviations).
Functional connectivity analyses
Amygdala connectivity
Across all groups, there was support for the engagement of the emotional arousal circuit characterized by positive covariation of the amygdala with both dorsal brainstem, including the LCC, and sgACC. However, covariate by group interactions indicated connectivity differences between IBS patients and HCs in the brainstem, amygdala, sgACC and aMCC (Table 3).
Table 3.
Covariation within a limbic stress circuit (amygdala, dorsal brainstem, 3 ACC regions).
| Cluster p | k | Voxel p (FWE) | T | X | y | z | ||
|---|---|---|---|---|---|---|---|---|
| Covariation with subgemal ACC positive covariation | ||||||||
| Amygdala | L | 0.041 | 30 | 0.024 | 3.49 | −18 | −6 | −16 |
| R | 0.020 | 66 | 0.031 | 3.37 | 26 | −8 | −18 | |
| Pregenual ACC | L | NS | NS | |||||
| R | 0.026 | 106 | 0.023 | 3.98 | 8 | 46 | −2 | |
| IBS>HC | ||||||||
| Dorsal brainstem | L | NS | NS | |||||
| R | 0.056 | 21 | 0.012 | 3.83 | 4 | −34 | −18 | |
| MCC | L | NS | NS | |||||
| R | 0.023 | 128 | 0.030 | 3.94 | 4 | 22 | 22 | |
| Positive covariation in the IBS group | ||||||||
| Dorsal brainstem | L | NS | NS | |||||
| R | 0.046 | 30 | 0.008 | 4.02 | 4 | −34 | −18 | |
| Amygdala | L | 0.037 | 35 | 0.019 | 3.60 | −18 | −6 | −14 |
| R | 0.037 | 33 | 0.019 | 3.60 | 22 | 0 | −24 | |
| Pregenual ACC | L | NS | NS | |||||
| R | 0.041 | 77 | 0.009 | 4.40 | 12 | 56 | −2 | |
| Negative covariation in the HC group | ||||||||
| MCC | L | NS | NS | |||||
| R | 0.030 | 110 | 0.050 | 3.70 | 2 | 34 | 32 | |
| Covariation with amygdala positive covariation (main effect) | ||||||||
| Dorsal brainstem | L | 0.054 | 21 | 0.036 | 3.34 | −6 | −34 | −14 |
| R | NS | NS | ||||||
| Subgenual ACC | L | 0.016 | 92 | 0.008 | 4.09 | −4 | 26 | −12 |
| R | 0.005 * | 173 | 0.002 * | 4.72 | 10 | 42 | −8 | |
| Pregenual ACC | L | NS | NS | |||||
| R | 0.014 | 153 | 0.007 | 4.49 | 6 | 46 | 0 | |
| IBS>HC group | ||||||||
| MCC | L | NS | NS | |||||
| R | 0.026 | 120 | 0.011 | 4.40 | 6 | 26 | 30 | |
| Positive covariation in the IBS group | ||||||||
| Subgenual ACC | L | 0.019 | 83 | 0.005 * | 4.32 | −4 | 26 | −10 |
| R | 0.027 | 57 | 0.013 | 3.81 | 10 | 42 | −10 | |
| Negative covariation in the HC group | ||||||||
| MCC | L | NS | NS | |||||
| R | 0.034 | 100 | 0.023 | 4.07 | 8 | 26 | 32 | |
significant after Bonferroni correction, 0.05/8 ROIs = 0.0063, k = # of voxels. Pla = placebo, FWE = family wise error corrected, ACC = anterior cingulate cortex, and MCC = mid cingulate cortex.
sgACC connectivity
IBS patients had more positive covariation than HCs between the sgACC and the dorsal brainstem. HCs, but not IBS patients, were characterized by negative correlations between activity aMCC and activity in both the amygdala and sgACC (Fig. 3).
Fig. 3.
Functional connectivity of relative glucose metabolism in other regions of interest with activity in sgACC, and amygdala. Depicts areas of covariation across all scans between metabolism in other ROIs and seed regions within (A) sgACC depicted on sagittal slices 4 mm to the right of the anterior commissure, and (B) amygdala depicted on sagittal slices 6 mm to the right of the anterior commissure (see Fig. 1 for format and abbreviations).
Correlation of brain response to YOH with early life trauma
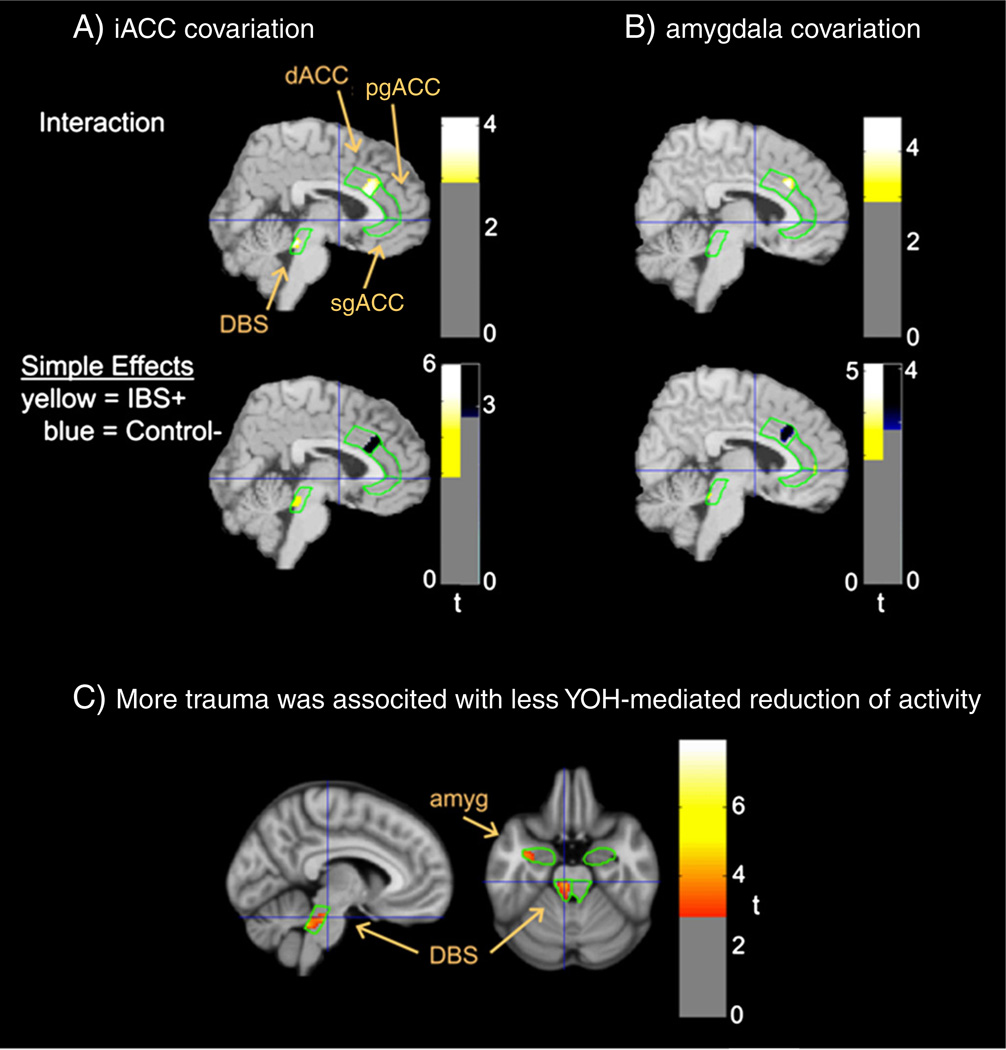
We assessed the relationship between early life trauma and the ability of YOH to downregulate activity within an emotional arousal circuit (amygdala, sgACC, dorsal brainstem). Group differences in covariation could not be adequately assessed because only six HCs were administered the trauma questionnaire. More trauma correlated with a smaller YOH-mediated reduction of activity in the left dorsal brainstem (spatial-extent volume-corrected p = 0.034; Family-wise error corrected maximum effect voxel p = 0.018 @ − 6, − 26, − 24, t = 4.20), with a similar trend in the left amygdala (p = 0.088; maximum effect voxel p = 0.065 @ − 30, − 6, − 24, t = 3.30). These relationships were restricted to the lateral but not medial amygdala and the pontine, but not midbrain portion of the dorsal brainstem (i.e. – LCC; see Fig. 4).
Fig. 4.
Covariation (p<0.005) of early life trauma with Yohimbine effects on relative glucose metabolism. Covariance between early life trauma and the ability of YOH, as compared to placebo, to downregulate relative glucose metabolism is depicted at coordinates −6, − 26, −24 (see Fig. 1 for format and abbreviations).
Discussion
In the current study, we show that pharmacological modulation of central NE circuits known to be involved in NE release, emotional arousal, attention and sympathetic nervous system activity differ between patients with IBS and HCs. These findings were not a consequence of comorbid anxiety disorders, since all patients were free of such diagnoses and rated state anxiety within the normal range.
Brain activity differences between groups were associated with differences in plasma NE and anxiety. In HCs, but not IBS, anxiety correlated positively with activity in limbic and right frontotemporal structures, but negatively with the left frontotemporal activity. Functional connectivity analysis of emotional arousal circuits revealed that only HCs showed expected negative correlations between ACC and limbic activity, consistent with compromised corticolimbic feedback inhibition in IBS (Mayer and Bushnell, 2009). These findings support previous reports of abnormalities in a central arousal circuit and its prefrontal modulation in IBS patients (Berman et al., 2008; Labus et al., 2008b).
Pharmacological modulation of the adrenergic system
We used the α2A receptor antagonist YOH and the α2A receptor agonist CLO to manipulate brain circuits involved in emotional arousal and associated autonomic and behavioral responses. Pharmacological manipulation of central α2A receptors with YOH and CLO have been used to study the NE modulation of brain activity in both humans and animals (Bremner et al., 1996a, 1996b). Although their effects are not specific for presynaptic α2A receptors, both drugs have been used to modulate presynaptic inhibitory α2A autoreceptors on LCC or projection neurons to stimulate (YOH) or inhibit (CLO) NE release, thereby regulating cortical/subcortical levels of NE. Since no peripheral visceral stimulus (e.g. bowel distension) was employed, it is unlikely that peripheral drug effects (Camilleri et al., 2003) can explain the behavioral and brain group differences we observed.
Peripheral and behavioral measures
There is evidence of increased sympathetic and sympathoadrenal activity in IBS patients from abnormal cardio autonomic responses (Adeyemi et al., 1999; Aggarwal et al., 1994), urine catecholamine levels (Heitkemper et al., 1996) and plasma NE levels (Wong et al., 2006). Consistent with these reports, IBS plasma NE levels were higher than HCs before pharmacological modulation, and after all drugs. IBS patients had greater YOH-induced increase in NE, consistent with greater reactivity of central sympathetic regulation. In contrast to plasma NE levels, no significant group differences were observed in blood pressure, despite the combined sample showing expected blood pressure increases after YOH, and decreases after CLO.
When viewed together, these findings support the concept of increased sympathetic tone and reactivity in IBS patients, as indexed by basal and YOH-induced plasma NE levels, without a corresponding abnormality in noradrenergic vascular regulation of systolic blood pressure (regulated by α1 and βARs). They also demonstrate that the oral doses used were sufficient to produce significant pharmacological effects without side effects. There were insignificant differences in self-rated anxiety and fatigue at baseline, but the greater YOH-induced increase in anxiety in patients and its correlation with the greater increase in plasma NE levels is also consistent with greater reactivity in IBS.
Drug effects on brain circuits involved in emotional arousal
Consistent with previous reports (Bremner et al., 1997) and with the presumed mechanism of NE on target neurons, YOH administration, as compared to PLA, reduced brain activity in MCC, while CLO increased activity in most ROIs. NE from ascending projection neurons acts on postsynaptic α2, αl and β receptors, with α2 receptors showing the highest affinity. Increasing extracellular NE inhibits spontaneous activity, presumably via α2 receptors, while sparing evoked responses to a variety of sensory stimuli, increasing the signal-to-noise ratio for specific stimuli (Ramos and Arnsten, 2007). This may be responsible for NE increase releasing CRF from limbic areas with noradrenergic input, including LCC and bed nucleus striae terminalis, thereby increasing anxiety and autonomic responses. By increasing the responsiveness of cortical attentional regions to visceral stimuli, it may also contribute to hypervigilance.
Compared to HCs, IBS patients had reduced YOH-mediated inhibition of brain activity in the ACC, amygdala, dorsal brainstem and pINS. The study was specifically designed not to record brain activity during a resting state, but during attentional vigilance to a validated oddball task. Although other interpretations are possible, these findings are consistent with reduced stimulatory effect of YOH on central NE release, possibly through reduced availability of presynaptic α2A receptors. Reduction of α2ARs has been reported in chronically stressed tree shrews, and this was associated with increased central NE (Fluegge et al., 2003). However, the findings may also reflect decreased postsynaptic inhibitory α2ARs, as previously suggested (Dinan et al., 1990).
Correlation of brain activity with subjective anxiety
The majority of ascending NE pathways originate from the pontine LCC, which in turn is a major recipient of afferents from limbic forebrain, including amygdala and PFC. Feed forward interactions between NE neurons in LCC and CRF – containing neurons in the hypothalamus and amygdala are implicated in central stress circuitry (Koob, 1999). Ascending NE pathways alter the responsiveness of emotional arousal and attentional mechanisms. Animal data suggest that ascending NE pathways modulate nociceptive response in subregions of the ACC (Vogt et al., 2009). Enhanced NE release from ascending projection neurons in vPFC and aMCC could therefore alter the ability of frontal regions to inhibit emotional arousal. Together, NE-related dysregulation of attentional and emotional circuits could play a role in the characteristic IBS features of hypervigilance and anxiety, by modulating the emotional arousal circuits (Mayer and Bushnell, 2009).
Our results suggest that the relationship of anxiety to the laterality of frontotemporal cortical activity is abnormal in IBS patients (Fig. 2). HCs displayed a strong positive covariation of self-rated anxiety with activity in the limbic system and right frontotemporal cortex, and an equally strong negative covariation with left frontotemporal cortex activity. These findings are consistent with prominent models of emotional processing postulating that positive emotions are associated with left anterior cortical activity and negative emotions with right anterior cortical and limbic activity (Coan and Allen, 2004; Davidson, 2002). There was no relationship between anxiety and corticolimbic activity or laterality in IBS patients. Fig. 2 suggests that cortical inhibition of the limbic stress circuit may be deficient in IBS, similar to a failure of depressed individuals to demonstrate left> right frontal cortex activation during cognitive downregulation of negative emotions (Johnstone et al., 2007). More generally, the absence of normal anterior cortical asymmetry of emotional regulation in both IBS and depression may be related to the asymmetric frontotemporal dendritic remodeling and suppression of cytogenesis that have been induced by chronic stress in animal models (Cullen et al., 2006; Czeh et al., 2008).
Evidence for altered connectivity in a central arousal circuit
Neuroanatomical and functional imaging evidences support the existence of an emotional arousal circuit involving the amygdala, sgACC and pgACC, in which projections from the pgACC to the amygdala provide negative feedback inhibition (Pezawas et al., 2005). The circuit receives multiple ascending noradrenergic inputs from LCC (Vogt et al., 2009), and inhibitory input from dorsal and vlPFC (McNaughton and Corr, 2004). Evidence for alterations in the connectivity of this arousal circuit in HCs based on the 5-HTTLPR polymorphism of the SERT (Kilpatrick et al., 2011), and in IBS patients (Labus et al., 2008a, 2011; Truong et al., 2008) has been reported. Herein, we confirmed our earlier findings that compared to HCs, IBS patients showed a lack of negative correlations between activity both in the amygdala and correlated sgACC with the aMCC/pgACC, consistent with a compromised negative feedback inhibition (Fig. 3). Supporting an extensive preclinical literature on the effects of aversive early life events on the expression of receptors and responsiveness of CRF/NE brain circuits (Meaney, 2001) (Caldji et al., 2000), we found that early life trauma was associated with smaller YOH-mediated reduction of brain activity in key components of the emotional arousal circuit, the LCC and amygdala (Fig. 4).
Some limitations of the study and considerations for future work included the following. We had a small sample size in the current study, and although an in depth structured clinical interview was conducted to exclude subjects with drug abuse and use histories, a formal drug screen test was not conducted. Additionally, an in depth clinical interview was conducted to rule out major psychiatric disorders, but due to the high prevalence of mood disorders such as anxiety and depression among IBS patients, it is possible that potential co-morbidities exist especially with anxiety spectrum disorders in this sample before the study began. Therefore, the results need to be interpreted accordingly. Another aspect to consider in future work would be to quantify plasma levels for the agonist clonidine and the antagonist yohimbine during the PET scan in order to better determine the effects of both in altering noradrenergic signaling in IBS.
Summary and conclusions
Congruent with earlier studies of PTSD (Bremner et al., 1996b), a syndrome frequently comorbid with IBS, our findings are consistent with alterations in the noradrenergic modulation of central arousal circuits in IBS patients. These alternations may be related to increased tonic firing of the LCC (Vogt et al., 2009), to a downregulation of pre and possibly postsynaptic α2A receptors (Fluegge et al., 2003) and/or a genetically determined alteration in the responsiveness of these receptors (Park and Camilleri, 2005). Persisting changes in the noradrenergic system due to adverse early life events, and/or chronic disease-related stress may be involved in these alterations.
Supplementary Material
Acknowledgments
Thanks to Alan Pan and George Amin at the Westside Diagnostic & Therapeutic Medical Center, Culver City, CA, and Cody Ashe-McNalley, UCLA, CNS, for their help with critical PET data recovery.
Abbreviations
- NE
noradrenergic
- HCs
healthy controls
- α2AR
α2-adrenoreceptor
- YOH
yohimbine
- CLO
clonidine
- PLA
placebo
- FDG
[18F]-fluoro-2-deoxy-d-glucose
- PET
positron emission tomography
- MCC
mid cingulate cortex
- sgACC
subgenual cingulate cortex
- pgACC
pregenual cingulate cortex
- INS
insula
- IBS
irritable bowel syndrome
- GI
gastrointestinal
- CRF
corticotropin releasing factor
- LCC
locus coeruleus complex
- BSQ
UCLA Bowel Symptom Questionnaire
- EPQR
Eysenck Personality Questionnaire-Revised
- VSI
Visceral Sensitivity Index
- HAD
Hospital Anxiety and Depression Index
- ETISF
Early Trauma Inventory Short Form
- SSR
Stress Symptom Rating scales
- SPM
Statistical Parametric Mapping software
- MNI
Montreal Neurological Institute
- ROI
region-of-interest
Footnotes
Funding sources: This research was supported in part by grants from the National Institute of Digestive Diabetes and Kidney Diseases (NIDDK) DK48351 (EAM), R24 AT002681 (EAM), and the US Department of Veterans Affairs VA Merit Review (BN).
Disclosures: No conflicts of interest exist.
References
- Adeyemi EO, Desai KD, Towsey M, Ghista D. Characterization of autonomic dysfunction in patients with irritable bowel syndrome by means of heart rate variability studies. Am. J. Gastroenterol. 1999;94:816–823. doi: 10.1111/j.1572-0241.1999.00861.x. [DOI] [PubMed] [Google Scholar]
- Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Weaver SA. Early life trauma and chronic pain. In: Mayer EA, Bushnell MC, editors. Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP Press; 2009. pp. 423–452. [Google Scholar]
- Arnsten AFT, Goldmanrakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus-monkey. Brain Res. 1984;106:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, FitzGerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD, Mayer EA. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist alosetron. Gastroenterology. 2002a;123:969–977. doi: 10.1053/gast.2002.35990. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Chang L, FitzGerald L, Antolin T, Camplone A, Mayer EA. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am. J. Gastroenterol. 2002b;97:2791–2797. doi: 10.1111/j.1572-0241.2002.07024.x. [DOI] [PubMed] [Google Scholar]
- Berman S, Suyenobu B, Naliboff BD, Bueller J, Stains J, Wong H, Mandelkern M, Mayer EA. Differential noradrenergic (NE) modulation of brain metabolic and electrical activity in IBS patients and healthy controls. Gastroenterology. 2007;132(A-726) [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma EY, de Jongh R, Olivier B, Groenink L. Fear-potentiated startle, but not light-enhanced startle, is enhanced by anxiogenic drugs. Pharmacol. Biochem. Behav. 2010;96:24–31. doi: 10.1016/j.pbb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney SD. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat related posttraumatic stress disorder. Ach. Gen. Psychiatry. 1997;54:246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J. Nerv. Ment Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Kim DY, McKinzie S, Kim HJ, Thomforde GM, Burton DD, Low PA, Zinsmeister AR. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2003;1:111–121. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]
- Chang L, Mayer EA, Johnson T, FitzGerald L, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000a;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]
- Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, Bernstein CN, Saba L, Naliboff B, Anton PA, Matin K. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000b;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coull JT, Buchel C, Friston KJ, Frith CD. Noradrenergically mediated plasticity in a human attentional neuronal network. Neurolmage. 2000;11(822–822) doi: 10.1006/nimg.1999.0513. [DOI] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br. J. Psychiatry. 2006;188:26–31. doi: 10.1192/bjp.bp.104.008169. [DOI] [PubMed] [Google Scholar]
- Czeh B, Perez-Cruz C, Fuchs E, Flugge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter? Behav. Brain Res. 2008;190:1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol. Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Dickhaus B, Mayer EA, Firooz N, Stains J, Conde F, Olivas TI, Fass R, Chang L, Mayer M, Naliboff BD. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am. J. Gastroenterol. 2003;98:135–143. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Barry S, Ahkion S, Chua A, Keeling PW. Assessment of central noradrenergic functioning in irritable bowel syndrome using a neuroendocrine challenge test. J. Psychosom. Res. 1990;34:575–580. doi: 10.1016/0022-3999(90)90032-y. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire: (EPQ-R Adult) Comprising the EPQ-Revised (EPQ-R) and EPQ-R Short Scale. San Diego: Educational and Industrial Testing Service; 1994. [Google Scholar]
- Farhadi A, Bruninga K, Fields J, Keshavarzian A. Irritable bowel syndrome: an update on therapeutic modalities. Expert Opin. Invest Drugs. 2001;10:1211–1222. doi: 10.1517/13543784.10.7.1211. [DOI] [PubMed] [Google Scholar]
- Fluegge G, van Kampen M, Mijnster MJ. Perturbations in brain monomamine systems during stress. Cell Tissue Res. 2003;315:1–14. doi: 10.1007/s00441-003-0807-0. [DOI] [PubMed] [Google Scholar]
- Flugge G, van Kampen M, Meyer H, Fuchs E. Apha2A and alpha2C-adrenoceptor regulation in the brain: alpha2A changes persist after chronic stress. Eur. J. Neurosci. 2003;17:917–928. doi: 10.1046/j.1460-9568.2003.02510.x. [DOI] [PubMed] [Google Scholar]
- Heitkemper M, Jarrett M, Cain K, Shaver J, Bond E, Woods NF, Walker E. Increased urine catecholamines and Cortisol in women with irritable bowel syndrome. Am. J. Gastroenterol. 1996;91:906–913. [PubMed] [Google Scholar]
- Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, Kelleher DL, Tillisch K, Naliboff BD, Mayer EA. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an –emotional-arousal circuit during expectation of abdominal pain. J. Neurosci. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Clarke G, Quigley EMM, Groeger JA, Dinan TG, Cryan JF. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 2012;36:310–340. doi: 10.1016/j.neubiorev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, Bueller JA, Suyenobu B, Jarcho JM, McRoberts JA, Niesler B, Mayer EA. The HTR3A polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943–1951. doi: 10.1053/j.gastro.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Labus J, Bolus R, Chang L, Wildund I, Naesdal J, Mayer E, Naliboff B. The visceral sensitivity index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol. Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the Visceral Sensitivity Index. Psychosom. Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Hamaguchi T, Mizuno T, Kano M, Fukudo S. 5-Httlpr gene polymorphism modulates activity and connectivity within an emotional arousal network of healthy control subjects during visceral pain. Gastroenterology. 2008a;134(A-121) [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neurolmage. 2008b;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TO, Evers EA, Backes WH, Brummer RJ, van Nieuwenhoven MA. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011;60:1196–1203. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky P, Mayer EA, Saper CB. The Biological Basis for Mind Body Interactions. Elsevier, Amsterdam; 2000. Long-term Behavioral and Neuroendocrine Adaptations to Adverse Early Experience; pp. 81–103. [DOI] [PubMed] [Google Scholar]
- Lim EP, Tan CH, Jay TM, Dawe GS. Locus coeruleus stimulation and noradrenergic modulation of hippocampo-prefrontal cortex long-term potentiation. Int. J. Neuropsychopharmacol. 2010;13:1219–1231. doi: 10.1017/S1461145709991131. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr. Pharm. Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Bushnell MC. Functional pain disorders: time for a paradigm shift? In: Mayer EA, Bushnell MC, editors. Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP Press; 2009. pp. 531–565. [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/ anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meyerbroeker K, Powers MB, van Stegeren A, Emmelkamp PMG. Does yohimbine hydrochloride facilitate fear extinction in virtual reality treatment of fear of flying? A randomized placebo-controlled trial. Psychother. Psychosom. 2012;81:29–37. doi: 10.1159/000329454. [DOI] [PubMed] [Google Scholar]
- Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- Naliboff BNRJL. Anxiety in functional pain disorders. In: Mayer EA, Bushnell DM, editors. Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP Press; 2009. pp. 185–214. [Google Scholar]
- Naliboff BD, Benton D, Solomon GF, Morley JE, Fahey JL, Bloom ET, Makinodan T, Gilmore SL. Immunological changes in young and old adults during brief laboratory stress. Psychosom. Med. 1991;53:121–132. doi: 10.1097/00006842-199103000-00002. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MI, Camilleri M. Genetics and genotypes in irritable bowel syndrome: implications for diagnosis and treatment. Gastroenterol. Clin. N. Am. 2005;34:305–317. doi: 10.1016/j.gtc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol. Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiol. Learn. Mem. 2011;96:263–271. doi: 10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology. 2012;37:1204–1215. doi: 10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Nam B, Bueller J, Smith S, Suyenobu B, Siffert J, McKelvy J, Naliboff B, Mayer EA. Neurokinin-1-receptor antagonism decreases anxiety and emotional arousal circuit response to noxious visceral distension in women with irritable bowel syndrome: a pilot study. Aliment. Pharmacol. Ther. 2012;35:36–367. doi: 10.1111/j.1365-2036.2011.04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong TT, Naliboff BD, Chang L. Novel techniques to study visceral hypersensitivity in irritable bowel syndrome. Curr. Gastroenterol. Rep. 2008;10:369–378. doi: 10.1007/s11894-008-0071-2. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunction. Trends Pharmacol. Sci. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- Verdu B, Decosterd I, Buclin T, Stiefel F, Berney A. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611–2632. doi: 10.2165/0003495-200868180-00007. [DOI] [PubMed] [Google Scholar]
- Vianna E, Labus JS, Berman SM, Suyenobu BY, Jarcho J, Tillisch K, Naliboff BD, Mayer EA. Increased allocation of cognitive resources for selective attention in IBS patients. Gastroenterology. 2009;136(A17-A17) [Google Scholar]
- Vogt BA, Aston-Jones G, Vogt LJ. Shared norepinephrinergic and cingulate circuits, nociceptive and allostatic interactions, and models of functional pain and stress disorders. In: Vogt BA, editor. Cingulate Neurobiology and Disease. New York: Oxford University Press; 2009. pp. 467–498. [Google Scholar]
- Welch BL. The generalization of students' problem when several different population variances are involved. Biometrika. 1947;34(1–2):28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Lirinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr., DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sri. U. S. A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HY, Mayer EA, Naliboff BD, Dickhaus B, Olivas TI, Ameen VZ, Reinholdt J, Chang L. Evidence for alterations in central autonomic activity during sleep in women with diarrhea-predominant irritable bowel syndrome (IBS-D) Gastroenterology. 2006;130(A-292) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.